2026
New review paper on misophonia and tinnitus published in Neuroscience and Biobehavioral Reviews

We are excited to share our latest review paper just published in Neuroscience and Biobehavioral Reviews. This is first comprehensive review discussing how misophonia and tinnitus are neurobiologically connected. Millions of people struggle with these conditions worldwide, yet we still lack precise diagnostic tools and treatments.
These conditions seem different (misophonia = hatred of specific sounds like chewing; tinnitus = phantom ringing in ears), but they share many similarities in how the brain processes them. Both show hyperactivity in auditory-limbic circuits and heightened stress responses, though they also have differential mechanisms explaining their emergence or progress.
In this paper, we’re proposing a new neurobiological framework. The Sensory-Salience Dysregulation model!! This model aims to explain how both conditions arise from similar brain mechanisms involving sensory overload, salience network dysfunction, and autonomic dysregulation.
Dr. Zanos Appointed to Editorial Board of the Neuropharmacology journal

We are pleased to announce that Dr. Zanos, Director of the Translational Neuropharmacology Lab, has been appointed as an Editorial Board Member of Neuropharmacology, one of the leading journals in the field of neuroscience and pharmacology.
Neuropharmacology, published by Elsevier, is a premier international journal that publishes high-impact research on the effects of drugs on the nervous system. The journal covers a broad range of topics including molecular, cellular, and behavioral neuropharmacology, making it an essential publication for researchers advancing our understanding of brain function and therapeutics.
As an Editorial Board Member, Dr. Zanos will contribute to the peer review process and help shape the direction of neuropharmacology research by evaluating cutting-edge manuscripts in his areas of expertise. This appointment recognizes Dr. Zanos’s significant contributions to the field and his standing as a leader in translational neuropharmacology research.
This role will further strengthen our lab’s connections to the broader scientific community and enhance our engagement with the latest developments in neuropharmacology.
Zanos lab publishes a short review article on Major Depressive Disorder in Trends in Molecular Medicine
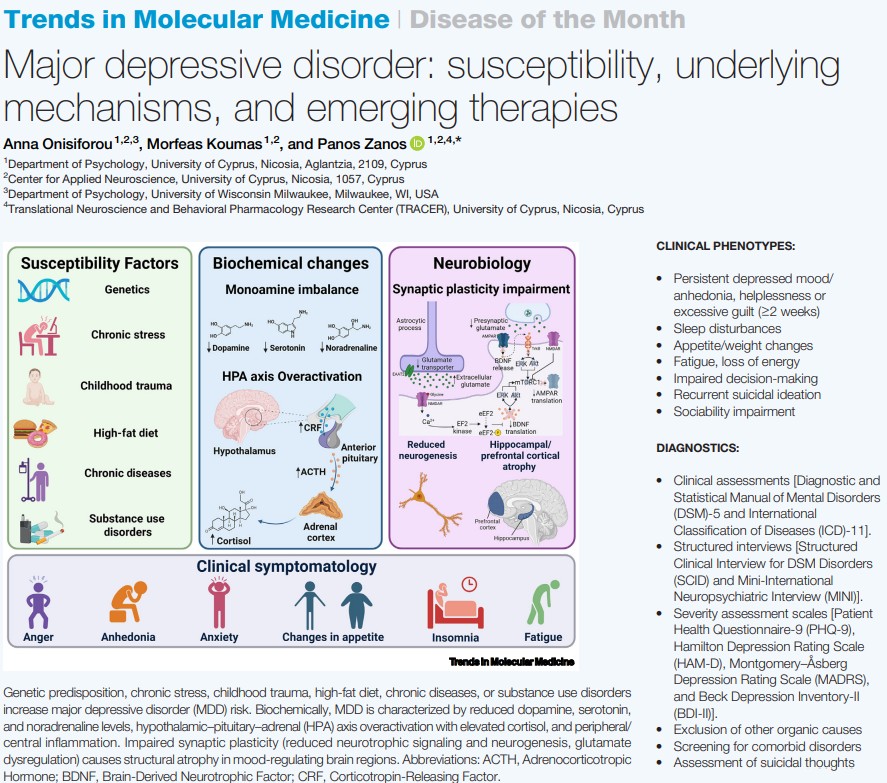
The Zanos Lab is pleased to announce the publication of a short review article in the Disease of the Month series of Trends in Molecular Medicine. The article, titled “Major depressive disorder: susceptibility, underlying mechanisms, and emerging therapies,” is authored by Dr. Anna Onisiforou, Mr. Morfeas Koumas, and the lab director, Dr. Panos Zanos.
This visually engaging overview provides a synthesis of current understanding of major depressive disorder, from genetic susceptibility and neurobiological mechanisms to diagnostic approaches and therapeutic interventions. The review aims to address the complex etiology of major depressive disorder by noting how genetic predisposition, chronic stress, childhood trauma, dietary factors, and substance use disorders increase vulnerability to depression. Key biochemical hallmarks are elucidated, including monoamine imbalances, hypothalamic-pituitary-adrenal axis overactivation, peripheral and central inflammation, and impaired synaptic plasticity leading to structural atrophy in mood-regulating brain regions. The article provides comprehensive coverage of diagnostic approaches, clinical phenotypes, and the multidimensional nature of depressive symptomatology.
A focus of this article is dedicated to the therapeutic landscape, discussing traditional pharmacotherapies alongside their limitations, including delayed onset of effects, modest remission rates, and significant adverse effects. The review highlights emerging therapeutic strategies for treatment-resistant depression, including ketamine and esketamine as rapid-acting antidepressants, transcranial magnetic stimulation, psychedelic-assisted therapies with psilocybin, and anti-inflammatory agents. These emerging therapies represent a paradigm shift moving beyond the monoamine hypothesis to address synaptic plasticity and neuroinflammation. The review concludes by noting the potential for precision medicine approaches using inflammatory markers and genetic data to guide personalized treatment selection.
To read the full article, please visit: https://www.sciencedirect.com/science/article/pii/S1471491425002904?dgcid=author
2025
Dr. Zanos appointed as science communication coordinator for European psychedelics research network
We are pleased to announce that Dr. Zanos has been appointed as Science Communication Coordinator for COST Action CA24130, titled “Psychedelic renaissance: turn on, tune in and drop in” (PSY-NET). This European research network brings together scientists, clinicians, industry partners, and regulatory bodies to advance research on psychedelics for therapeutic applications. Dr. Zanos’ appointment to this key position recognizes his expertise in translational neuropharmacology and his commitment to bridging the gap between cutting-edge neuroscience research and broader scientific, clinical, and public audiences.
COST Action CA24130 represents a major European initiative addressing the resurgence of psychedelic research for treating mental health disorders. It is a comprehensive translational project encompassing chemistry and biophysics, preclinical and clinical studies, advances in neuroimaging, data sharing and databases, and policy-making considerations. Given the current stagnation in developing new treatments based on traditional pharmacological mechanisms in neuroscience, psychedelics such as psilocybin, LSD, DMT, and MDMA have opened up a rapidly expanding field of research into consciousness, mental disorders, and their treatment. These compounds have already shown efficacy in treating depression, and recent advances in neuroimaging have enabled detailed understanding of the processes behind their unique therapeutic effects. The PSY-NET action aims to advance this field through coordinated European research efforts while also working to shift policy at the European level regarding the availability of these compounds in clinical and preclinical settings.
As Science Communication Coordinator, Dr. Zanos will hold a mandatory position within the COST Action responsible for coordinating the implementation of a comprehensive communication and dissemination strategy to enable the network to achieve its objectives and increase its visibility across Europe and beyond. This strategic role involves managing multiple communication channels including websites, social media platforms, and traditional media engagement to effectively communicate the Action’s activities and findings to diverse audiences including scientific experts, healthcare professionals, policy makers, regulatory agencies, and the general public. The Science Communication Coordinator plays a crucial role in bridging the gap between fundamental research and its practical applications, ensuring that groundbreaking discoveries about the therapeutic effects of psychedelics reach the world’s most prestigious journals while also being translated into accessible information for stakeholders and the public.
Dr. Anna Onisiforou secures €100,000 ERC Vision funding for Multiple Sclerosis research

We are pleased to announce that Dr. Anna Onisiforou, Senior Scientist and Head of the AI & Systems Bioinformatics Unit of our lab, has secured €100,000 in funding through the ERC Vision program of the Research and Innovation Foundation of Cyprus. This outstanding achievement recognizes Dr. Onisiforou’s innovative research and her leadership in developing computational methodologies for understanding virus-mediated pathogenic mechanisms in complex neurological diseases.
The funded research project, acronymed ImmUnoForecastMS, leverages artificial intelligence (AI), systems bioinformatics, and experimental approaches to identify and prioritize therapeutic candidates capable of mitigating the pathogenic effects of Epstein-Barr virus-induced immune dysregulation in multiple sclerosis. By applying advanced computational methods to large-scale biological datasets, the project will identify molecular patterns and therapeutic targets that might not be apparent through traditional analytical methods. This integrative strategy has the potential to reveal novel therapeutic candidates that can specifically address the virus-mediated components of multiple sclerosis pathogenesis, potentially leading to more effective and targeted treatment approaches for patients.
The ImmUnoForecastMS project builds directly on a substantial body of original work that Dr. Onisiforou has initiated, developed, and advanced over many years, helping to shape current understanding of how viral infections contribute to disease development. This funding underscores the significance and originality of her contributions to the field and reflects the Research and Innovation Foundation’s commitment to supporting cutting-edge research that addresses critical health challenges.
We congratulate Dr. Onisiforou on this significant achievement and look forward to the important discoveries that will emerge from the ImmUnoForecastMS project.
Zanos Lab research on ketamine’s therapeutic mechanisms in depression and Opioid Use Disorder was presented in a seminar at the University of Haifa

The Zanos Lab presented research on the neurobiological mechanisms underlying ketamine’s efficacy in treating both depression and substance use disorders. Dr. Zanos, Director of the Translational Neuropharmacology Lab, delivered a talk detailing the lab’s multifaceted approach to understanding how ketamine and its metabolites can address two of the most pressing public health challenges of our time: depression and opioid use disorder (OUD).
Depression and opioid use disorder represent significant global health burdens with limited effective treatment options, making the development of novel therapeutic approaches critically important. The Zanos Lab has been systematically investigating the therapeutic potential and mechanistic basis of ketamine and its metabolites using an integrative research strategy that combines behavioral, electrophysiological, and molecular approaches in preclinical models. Through extensive studies in mice, the lab has uncovered a surprising and paradigm-shifting finding: contrary to the prevailing view that ketamine works primarily through NMDA receptor blockade, the research demonstrates that NMDA receptor activation, rather than inhibition, is actually essential for its rapid antidepressant effects. The lab’s work has revealed that ketamine exhibits an inverted U-shaped dose-response relationship, and its antidepressant-like properties, hippocampal AMPA receptor upregulation, and metaplasticity induction are abolished when NMDA receptors are blocked prior to ketamine administration. This discovery extends to ketamine’s key metabolite, (2R,6R)-hydroxynorketamine (HNK), and other rapid-acting antidepressants, all of which require NMDA receptor signaling for their therapeutic actions. Importantly, the research has identified the GluN2A subunit of the NMDA receptor as being both necessary and sufficient for mediating these antidepressant effects, providing a specific molecular target for future therapeutic development.
Building on these mechanistic insights, the Zanos Lab has extended its preclinical investigations to opioid use disorder, demonstrating that (2R,6R)-HNK shows promise in addressing multiple aspects of this devastating condition. In preclinical OUD models, (2R,6R)-HNK effectively countered morphine conditioning in stress-vulnerable mice, prevented withdrawal symptoms, and alleviated anhedonia, anxiety, and cognitive deficits that emerge during protracted abstinence. Perhaps most significantly for long-term recovery outcomes, (2R,6R)-HNK enhanced the extinction of opioid conditioning, blocked stress-triggered relapse, and reduced subsequent opioid consumption. These therapeutic effects appear to be mediated, at least in part, by the compound’s ability to restore disrupted cortical high-frequency EEG oscillations that are characteristic of the addicted brain state. The preclinical findings reveal a convergent therapeutic mechanism whereby (2R,6R)-HNK promotes GluN2A-NMDAR-dependent synaptic plasticity to provide rapid antidepressant effects while simultaneously addressing the negative affective states and relapse vulnerability that characterize opioid use disorder.
Translating these promising preclinical findings to human populations represents the critical next step in developing ketamine-based therapies for opioid use disorder. The Zanos Lab is currently conducting the PROUD study (Prevention of Relapse in Opioid Use Disorder), a Phase II randomized, double-blind, placebo-controlled clinical trial examining ketamine’s efficacy in patients with opioid use disorder who are undergoing opioid substitution treatment. This human study, funded by the Research and Innovation Foundation of Cyprus is investigating whether a two-week regimen of subanesthetic doses of ketamine (0.5 mg/kg) can improve treatment retention, reverse comorbid negative affective behaviors including depression and anhedonia, and prolong abstinence in patients receiving buprenorphine/naloxone maintenance therapy. The study enrolls 60 adults aged 18-65 who meet criteria for moderate-to-severe opioid use disorder and are experiencing depressive symptoms. Beyond assessing clinical outcomes, the PROUD study is also examining novel biomarkers that may predict vulnerability to relapse, including emotion regulation ability measured through heart rate variability, stress response biomarkers such as cortisol and catecholamines, and changes in neural activity measured via EEG spectral analysis. Patients are followed for nine months after their last ketamine infusion to assess long-term effects on relapse prevention. This comprehensive approach aims to not only establish ketamine’s clinical efficacy but also to identify predictive markers that could personalize treatment strategies for individuals at highest risk of relapse during the challenging period of protracted abstinence.
Our Ph.D. student, Despina Melanthiou, featured in national newspaper article on Misophonia research

We are pleased to announce that Despina Melanthiou, a PhD student in the Zanos Lab, was featured in an article published in Politis newspaper on December 14, 2025, discussing her research on misophonia and its neurological underpinnings.
The article, titled “When Everyday Sounds Become Pathologically Unbearable,” explores misophonia as a neurological condition that affects approximately 10-20% of the general population. Despina’s work focuses on understanding how common everyday sounds, such as chewing, breathing, or keyboard typing, can trigger intense negative emotional and physiological responses in individuals with this condition. While these sounds are typically innocuous to most people, those suffering from misophonia experience them as unbearable, often leading to significant distress and impairment in daily functioning.
Through her research at the University of Cyprus, Ms. Melanthiou will be contributing to the scientific understanding of misophonia’s neurological mechanisms and exploring contemporary approaches to diagnosis and assessment. The article highlights how this condition, though increasingly recognized in clinical and research settings, requires further investigation to develop effective therapeutic interventions. Her work examines the complex interplay between auditory processing, emotional regulation, and neural connectivity that characterizes misophonic responses.
The publication of this article in a major national newspaper represents an important contribution to public awareness and education about misophonia. By bringing scientific research findings to a broader audience, we will be helping to reduce stigma and misconceptions surrounding this often-misunderstood condition. Many individuals with misophonia have historically been dismissed or misdiagnosed, and increased public knowledge can lead to better recognition, understanding, and support for those affected.
Translational Neuropharmacology Lab awarded €535,000.00 Horizon Europe funding
The Translational Neuropharmacology Lab at the University of Cyprus has been awarded funding for the EXPOSIGNALZ HOP-ON project under the Horizon Europe HORIZON-WIDERA-2025-03 call. The proposal received the maximum evaluation score of 15.00/15.00, with perfect scores across all three assessment criteria: Excellence, Impact, and Quality and Efficiency of Implementation.
This 48-month research initiative, conducted in partnership with the Institut National de la Santé et de la Recherche Médicale (INSERM) in France, will investigate the causal mechanisms linking environmental pollutant exposure to neurodegeneration and Alzheimer’s disease. Building on EXPOSIGNALZ’s identification of neurotoxic compounds and pro-amyloidogenic chemical signatures, our lab will employ cutting-edge systems neuroscience approaches to examine how exposure to environmentally relevant toxicants perturbs key memory circuits in mouse models. The research will utilize advanced methodologies including ex vivo electrophysiology, in vivo fiber photometry, optogenetic interventions, and electroencephalography to identify circuit-level dysfunctions and test potential therapeutic interventions.
The project aims to generate the first translational biomarkers of pollutant-induced circuit dysfunction, define critical developmental windows of susceptibility, and evaluate possible rescue interventions. This widening action will establish our lab as a reference center for environmental neurotoxicity research in Southeast Europe while contributing to evidence-based brain health protection policies across the European Research Area.
Our Ph.D. student Morfeas Koumas authors public article on Opioid Use Disorder

We’re pleased to share that Morfeas Koumas, PhD student in the Zanos Lab, has published an article in Neolaia (Youth), a Cyprus-based journal, as part of the dissemination efforts for our PROUD study. The article, titled “Opioid Use Disorder: A Growing Public Health Challenge,” provides an accessible overview of opioid use disorder and discusses emerging therapeutic approaches, with a particular focus on ketamine’s potential as a novel treatment option. In the article, Morfeas explains how opioid use disorder affects an estimated 1.7 million people in the European Union who received treatment in 2023, and in Cyprus specifically, approximately 1,200 individuals use opioids, with opioid-related deaths representing 80-90% of all drug-related fatalities.
Morfeas discusses how while current pharmacotherapies like methadone, buprenorphine, and naltrexone are effective for maintaining abstinence, they have limited effectiveness in addressing the underlying emotional symptoms and cognitive impairments that accompany opioid withdrawal. He highlights ketamine as a promising therapeutic approach, explaining how this rapid-acting antidepressant shows potential in reducing withdrawal symptoms during opioid cessation, decreasing cravings during both acute and prolonged abstinence, and addressing comorbid depression—a major risk factor for relapse. The article emphasizes that ketamine shows enhanced effectiveness when combined with psychotherapy, offering a dual benefit by helping both with opioid dependence and co-occurring depressive symptoms.
The article features our ongoing PROUD study at the University of Cyprus, led by Dr. Panos Zanos in collaboration with the “Gefyra” (Bridge) substance substitution unit. This double-blind, placebo-controlled study represents Cyprus’s first Phase 2 pharmaceutical-interventional clinical trial and is investigating whether ketamine can simultaneously address comorbid depression and reduce relapse rates in individuals with opioid use disorder. Alongside the clinical trial, Morfeas explains how our team is conducting preclinical research to discover novel pharmacotherapies that replicate ketamine’s therapeutic benefits while minimizing its side effects, including abuse potential and dissociative symptoms.
Scientific symposium: Addressing opioid addiction through interdisciplinary approaches

The Zanos Lab and the PROUD project team successfully hosted a scientific symposium titled “Addressing Opioid Addiction: Interdisciplinary Approaches” on December 5 at the University of Cyprus Library Amphitheater LRC012. This symposium brought together leading experts from psychiatry, clinical psychology, neuropharmacology, and community advocacy to discuss comprehensive approaches to understanding and treating opioid use disorder, one of the most pressing public health challenges facing our society today.
The symposium featured five distinguished speakers who presented diverse perspectives on opioid addiction, reflecting the multifaceted nature of this complex condition. Dr. Evanthia Bella, Psychiatrist and Visiting Lecturer in Psychiatry at the Medical School of the University of Cyprus, opened the symposium with a presentation on clinical assessment and therapeutic challenges in opioid addiction, providing essential insights into the diagnostic and treatment landscape. Following this, Dr. Dimos Fotopulos, Addiction Psychiatrist and Scientific Coordinator of Opioid Agonist Therapy Structures at the Mental Health Services Directorate of Cyprus State Health Services Organization, discussed opioid agonist therapy protocols and safety considerations in clinical practice, offering practical perspectives from the frontlines of addiction treatment. Dr. Marios Adonis, Associate Professor of Clinical Psychology and Chair of the Department of Social Sciences at the University of Nicosia, presented on psychosocial interventions in opioid and other substance addictions, highlighting the crucial role of psychological and social factors in recovery and rehabilitation.
Dr. Panos Zanos, Assistant Professor of Neuropharmacology in the Department of Psychology at the University of Cyprus and Principal Investigator of the PROUD study, presented on ketamine as an innovative pharmaceutical option for opioid addiction. This presentation discussed the translational neuropharmacology research underpinning the PROUD clinical trial, including preclinical findings on ketamine’s mechanisms of action and its potential to address multiple domains of opioid use disorder, from promoting treatment retention to reversing comorbid affective disorders and maintaining abstinence. The presentation bridged basic neuroscience research with clinical applications, demonstrating how mechanistic insights into NMDA receptor-dependent synaptic plasticity can inform novel therapeutic strategies for this challenging condition.
The symposium also featured Ms. Maria Diplarou, Social and Developmental Psychologist and Representative of the Organization of Friends and Relatives of Addicted Persons (OFSEA), who spoke on the crucial importance of the immediate environment and family support systems for individuals with substance use disorders. Her presentation provided valuable insights into the lived experiences of families affected by addiction and the essential role that community-based support plays in long-term recovery. This diverse panel of speakers reflected the symposium’s core theme: that addressing opioid addiction effectively requires an interdisciplinary approach that integrates clinical psychiatry, psychological interventions, neuropharmacological innovations, and community-based support systems. The event concluded with an engaging discussion period, providing an opportunity for attendees to engage with the speakers and explore the synergies between different therapeutic approaches.
The symposium was moderated by Dr. Georgia Panayiotou, Dean of the School of Graduate Studies at the University of Cyprus, and team member of the PROUD project, a clinical trial funded by the Research and Innovation Foundation of Cyprus. The PROUD project represents a pioneering effort to translate cutting-edge neuroscience research into clinical practice, examining whether ketamine can improve outcomes for individuals undergoing opioid substitution therapy. This symposium exemplified the collaborative spirit and translational focus that characterizes the PROUD project, bringing together researchers, clinicians, and community advocates to advance our collective understanding of opioid addiction and develop more effective, evidence-based treatment approaches.
Zanos lab presents PROUD study findings at NeuroGeorgia 2025 international neuroscience conference

The Zanos Lab recently presented groundbreaking findings from the PROUD clinical trial at the NeuroGeorgia 2025 International Neuroscience Conference. The presentation, delivered by the PROUD research team, showcased the first randomized controlled trial results examining ketamine’s antidepressant efficacy and underlying neurobiological mechanisms in individuals with opioid use disorder during abstinence maintenance. This pioneering work addresses a critical gap in treatment options for a highly vulnerable population facing both addiction and mental health challenges.
Opioid use disorder represents a global public health crisis, with depression co-occurring in approximately thirty to fifty percent of individuals receiving opioid substitution treatment. This comorbidity substantially worsens treatment outcomes, increases relapse risk, and elevates suicide mortality. Despite this critical clinical need, evidence-based pharmacological interventions addressing depression in opioid use disorder populations remain limited, particularly during the vulnerable abstinence maintenance phase when conventional antidepressants demonstrate delayed onset and modest efficacy. While ketamine has demonstrated rapid antidepressant effects in treatment-resistant depression, its therapeutic potential and mechanistic profile in opioid use disorder populations with comorbid depression remained unclear prior to the PROUD study.
The PROUD project represents the first double-blind, placebo-controlled, randomized clinical trial specifically examining ketamine’s efficacy, safety, and neurobiological mechanisms in this population. The study enrolled participants with opioid use disorder under supervised abstinence maintenance who presented with comorbid depressive symptoms, aged eighteen to sixty-five years. Participants received six intravenous infusions of ketamine at a dose of 0.5 mg/kg or saline placebo, administered over forty minutes across a two-week period. Depression severity was assessed using the Montgomery-Åsberg Depression Rating Scale at multiple time points including baseline, forty to eighty minutes post-infusion, twenty-four hours, two weeks, three months, and nine months post-infusion. The study incorporated comprehensive assessments of potential mechanistic factors, including heart rate variability as an index of autonomic and emotional regulation, stress biomarkers including plasma cortisol, adrenocorticotropic hormone, noradrenaline and adrenaline, synaptic plasticity markers such as brain-derived neurotrophic factor, and electroencephalography recordings to assess cortical neuronal activity changes at baseline, during infusion, and post-infusion.
The results presented at the conference revealed a delayed-onset antidepressant profile that was notably distinct from findings in major depression studies. Depression rating scale scores showed no immediate post-infusion group differences at the forty-minute time point, with therapeutic effects emerging at twenty-four hours and sustained through the two-week follow-up period. This unique temporal profile suggests that ketamine may exert its antidepressant effects through different mechanisms or kinetics in opioid use disorder populations compared to individuals with major depression alone. Preliminary mechanistic analyses examining heart rate variability, stress biomarkers, and synaptic plasticity markers revealed promising patterns that could potentially explain some of the unique temporal profile and sustained efficacy of ketamine in this population. These findings suggest that ketamine’s effects in opioid use disorder may involve distinct neurobiological pathways related to stress regulation, autonomic function, and synaptic remodeling.
The findings from the PROUD study suggest that ketamine may offer a novel therapeutic approach for treatment-resistant depression in opioid use disorder populations during abstinence maintenance, with a distinct efficacy profile that differs from its effects in primary depression. The delayed but sustained antidepressant response observed in the study has important implications for clinical implementation, suggesting that patients and clinicians should anticipate a different time course of therapeutic benefits compared to ketamine treatment for major depression. Ongoing analyses of the comprehensive neurobiological data collected in the study may identify biomarkers predicting treatment response in this vulnerable population, potentially enabling personalized treatment approaches that could optimize outcomes for individuals at highest risk of relapse during the challenging abstinence maintenance phase. This work represents a significant advance in addressing the unmet clinical need for effective treatments targeting the intersection of addiction and mental health disorders.
Misophonia Research Presented at the 15th International Conference of the Center for Applied Neuroscience
We are delighted to announce that our Ph.D. student, Despina Melanthiou, presented her research on misophonia at the 15th International Conference of the Center of Applied Neuroscience, held at the University of Cyprus.
Despina’s presentation showcased our ongoing investigation into the neurophysiological mechanisms underlying misophonia, a condition characterized by intense emotional and physical reactions to specific auditory triggers. Since 2023, our team has been working to identify reliable biomarkers that can objectively measure and characterize misophonic responses.
The research employs a comprehensive, multi-modal approach that integrates EEG to capture neural activity patterns, salivary biomarker analysis to measure stress-related hormones such as cortisol and alpha-amylase, and detailed behavioral assessments. By examining the complex interplay between auditory processing, limbic system activation, and autonomic nervous system responses, this work aims to establish objective diagnostic criteria and advance our understanding of sensory-emotional integration disorders.
This research has significant implications beyond misophonia itself, serving as a valuable model for understanding how the brain processes and responds to aversive sensory stimuli. The findings may inform the development of targeted interventions and contribute to broader knowledge about sensory processing dysfunctions and hypervigilance mechanisms.
Through this work, we continue to bring scientific rigor to a condition that has been historically understudied despite its substantial impact on quality of life for those affected.
Novel Computational Framework Reveals EBV’s Role in Multiple Sclerosis Presented at 15th International Conference of the Center for Applied Neuroscience
 We are proud to announce that our Senior Researcher Dr. Anna Onisiforou presented groundbreaking research on the role of Epstein–Barr virus (EBV) in Multiple Sclerosis (MS) at the 15th International Conference of the Center of Applied Neuroscience, held at the University of Cyprus.
We are proud to announce that our Senior Researcher Dr. Anna Onisiforou presented groundbreaking research on the role of Epstein–Barr virus (EBV) in Multiple Sclerosis (MS) at the 15th International Conference of the Center of Applied Neuroscience, held at the University of Cyprus.
Dr. Onisiforou’s presentation introduced VirTrack, an innovative computational framework developed in our laboratory that integrates experimentally validated EBV–host protein–protein interactions with clinical type–specific peripheral blood transcriptomes. This novel approach enables systematic investigation of how EBV influences different MS clinical types, including Clinically Isolated Syndrome (CIS), Relapsing Remitting MS (RRMS), Secondary Progressive MS (SPMS), and Primary Progressive MS (PPMS).
The research reveals that EBV engagement is highly clinical type–dependent. In early MS stages (CIS and RRMS), EBV targets approximately 13–18% of dysregulated genes, enriching for B-cell–related processes, Toll-like receptor signaling, and infection-like inflammatory pathways, while suppressing antiviral and NF-κB responses. Progressive clinical types showed fewer viral connections but exhibited distinct mechanistic shifts: SPMS was characterized by suppression of vascular and cardiac repair–associated pathways, whereas PPMS was dominated by upregulation of vacuolar and lysosomal remodeling processes.
The analysis identified a stable core of influential EBV proteins, with EBNA-LP consistently ranking highest, alongside BZLF1, BVLF1, LMP2, and BDLF4. These findings suggest that EBV shapes MS through dynamic, clinical type–specific perturbations, driving strong immunomodulation in early disease and selective cellular remodeling during progressive stages.
This work has important implications for stage-tailored therapeutic targeting, supporting the potential for early EBV-directed interventions and revealing possible links to vascular comorbidity in progressive MS types. Beyond MS, VirTrack offers a generalizable, systems-level framework for elucidating viral contributions across complex human diseases.
Dr. Zanos Moderated Symposium on Non-Invasive Neuromodulation at 15th International Conference of the Center for Applied Neuroscience

Dr. Zanos, served as moderator for the invited symposium session titled “Non-Invasive Neuromodulation: Current State and Future Directions” at the 15th International Conference of the Center for Applied Neuroscience, held at the University of Cyprus.
The session featured international speakers who are leading experts in the field of neuromodulation and brain stimulation:
- Professor Alexander T. Sack (Maastricht University) presented “Home-Based Brain Stimulation: Bridging Cognitive Research and Clinical Therapies”.
- Dr. Jelena Trajkovic (Maastricht University) presented “Network-Based Neuromodulation”.
- Professor Chris Baeken (Vrije Universiteit Brussel and Ghent University) presented “Accelerated rTMS Protocols in Psychiatric Clinical Research”.
- Dr. Stefanie De Smet (Ghent Experimental Psychiatry Lab) presented “Brain-State Dependence of Non-Invasive Brain Stimulation: Insights and Clinical Implications”.
The symposium provided a comprehensive overview of cutting-edge approaches in non-invasive brain stimulation, covering topics from cognitive neuroscience applications to psychiatric therapeutic interventions. The session facilitated important discussions on the future directions of neuromodulation research and its clinical translation.
Following the presentations, Dr. Zanos moderated an engaging panel discussion with all speakers, addressing questions from the audience and fostering dialogue on this rapidly evolving field.
PROUD Project Presented at the 15th International Conference of the Center for Applied Neuroscience

We are pleased to announce that our Ph.D. student Morfeas Koumas presented our “PROUD” project at the 15th International Conference of the Center of Applied Neuroscience, held at the University of Cyprus.
Morfeas’s poster presentation showcased our ongoing randomized, double-blind, placebo-controlled clinical trial investigating the efficacy of ketamine in treating negative affect and preventing relapse to opioid use during abstinence. The PROUD project (EudraCT: 2022-001997-70) represents an innovative approach to addressing a critical gap in opioid use disorder (OUD) treatment.
The study explores ketamine’s potential as a novel pharmacotherapy, leveraging its unique properties as a fast-acting antidepressant that produces robust effects from a single sub-anesthetic dose. The protocol involves six intravenous subanesthetic ketamine doses (0.5 mg/kg, 40-minute infusion) or placebo administered over two weeks. Additionally, the trial aims to identify potential biomarkers predicting treatment response through heart rate variability and EEG measurements during the first ketamine or placebo infusion.
The presentation highlighted the trial’s methodology and its potential impact on developing more effective interventions for individuals with OUD, a chronic relapsing disorder with high overdose mortality.
The PROUD project is funded by the Research and Innovation Foundation and conducted at the premises of the State Health Services Organization of Cyprus and more specifically at the “GEFYRA” (BRIDGE) Substitute Substance Unit.
Dr. Zanos was invited to give a talk at the Foundation for Research and Technology in Crete about our ongoing studies on ketamine and opioid use disorders

Dr. Panos Zanos was recently invited to present at the Foundation for Research and Technology (FORTH) in Crete, where he shared insights into the Zanos Lab’s pioneering work on ketamine and opioid use disorders. His presentation focused on the PROUD study, a groundbreaking clinical trial investigating the therapeutic potential of ketamine in individuals with opioid addiction—the first clinical trial of its kind ever conducted in Cyprus. This milestone represents a significant advancement in addiction medicine research on the island and reflects the lab’s commitment to addressing the opioid crisis through innovative, evidence-based interventions.
A key aspect of Dr. Zanos’s presentation centered on why ketamine represents a particularly promising therapeutic option for individuals struggling with opioid use disorder. Depression is highly prevalent among those with opioid addiction, with studies showing that the majority of individuals in treatment for opioid use disorder experience significant depressive symptoms. This comorbidity creates a devastating cycle: depression increases the risk of relapse, while continued opioid use exacerbates depressive symptoms. Traditional antidepressants often take weeks to show effects and have limited efficacy in this population, leaving a critical gap in treatment options during the vulnerable period of early abstinence.
Ketamine’s unique properties make it an ideal candidate for addressing this dual challenge. Unlike conventional antidepressants, ketamine produces rapid antidepressant effects—often within hours—which could provide immediate relief during the acute withdrawal and early abstinence phases when patients are most vulnerable to relapse. Additionally, emerging evidence suggests that ketamine may help restore neuroplasticity and disrupt maladaptive reward memories associated with drug use. The PROUD study aims to determine whether ketamine’s combined effects on both depressive symptoms and addiction-related neural circuitry can significantly reduce relapse rates and improve overall outcomes for individuals with opioid use disorder in Cyprus, potentially establishing a new standard of care for this population.
Our Ph.D. student, Morfeas Koumas awarded a FENS/IBRO-PERC Fellowship for advanced training in Barcelona

We are delighted to announce that our first-year Ph.D. student, Morfeas Koumas (BSc, MSc), has been awarded the FENS/IBRO-PERC Fellowship to conduct a six-week research exchange at the University of Barcelona. Morfeas will train under the mentorship of Dr. Jordi Bonaventura, an expert in addiction neuroscience and behavioral pharmacology.
This fellowship represents a crucial step forward for both Morfeas’s doctoral training and our lab’s research capabilities. During his stay in Barcelona, Morfeas will receive intensive hands-on training in intravenous self-administration protocols, a gold-standard technique for studying substance use disorders and relapse behaviors in preclinical models.
While our lab recently acquired ten IVSA chambers, this specialized expertise is not currently available in Cyprus, making this international training opportunity essential for advancing our research program. By acquiring this expertise, Morfeas will establish our lab as the first in Cyprus with operational IVSA capabilities, enabling us to conduct high-translational addiction research and strengthen our competitiveness for future collaborative projects across Europe.
We are immensely proud of Morfeas’s achievement and look forward to the new research directions this training will enable.
Dr. Andria Michael presents research on ketamine-based therapies for opioid relapse prevention
Dr. Andria Michael, a postdoctoral researcher in the Zanos Lab, recently represented our laboratory at the 13th International Multithematic Scientific Bio-Medical Congress in Cyprus, at the European University Cyprus School of Medicine. Her presentation highlighted groundbreaking work on novel pharmacotherapies for preventing relapse to opioid use disorders during abstinence. The research focuses on ketamine, a compound that has shown promising potential in disrupting the neurobiological mechanisms that drive individuals back to drug use after periods of abstinence.
Dr. Michael detailed our ongoing Phase II randomized controlled clinical trial examining the efficacy of intravenous ketamine administration in patients undergoing opioid abstinence. This rigorous clinical study represents a critical step in translating preclinical findings into real-world therapeutic interventions for individuals struggling with opioid use disorder. By carefully monitoring patients’ responses to controlled ketamine treatments, the trial aims to establish both the safety profile and therapeutic potential of this approach in preventing relapse during the vulnerable period of early abstinence.
Beyond the clinical trial, Dr. Michael also explained our parallel efforts using animal models to identify next-generation compounds that could replicate ketamine’s beneficial effects while minimizing its known side effects, such as dissociation and potential for misuse. This translational research approach allows the lab to screen and optimize novel pharmacological agents before advancing them to human studies, potentially paving the way for safer and more effective treatments for opioid use disorder. Through this comprehensive research program, combining clinical trials with mechanistic animal studies, the Zanos Lab continues to advance our understanding of addiction neurobiology and develop innovative therapeutic strategies to combat the opioid crisis.
New pre-print: VirTarget framework reveals how Multiple Sclerosis immunotherapies engage with Epstein-Barr Virus-driven disease mechanisms
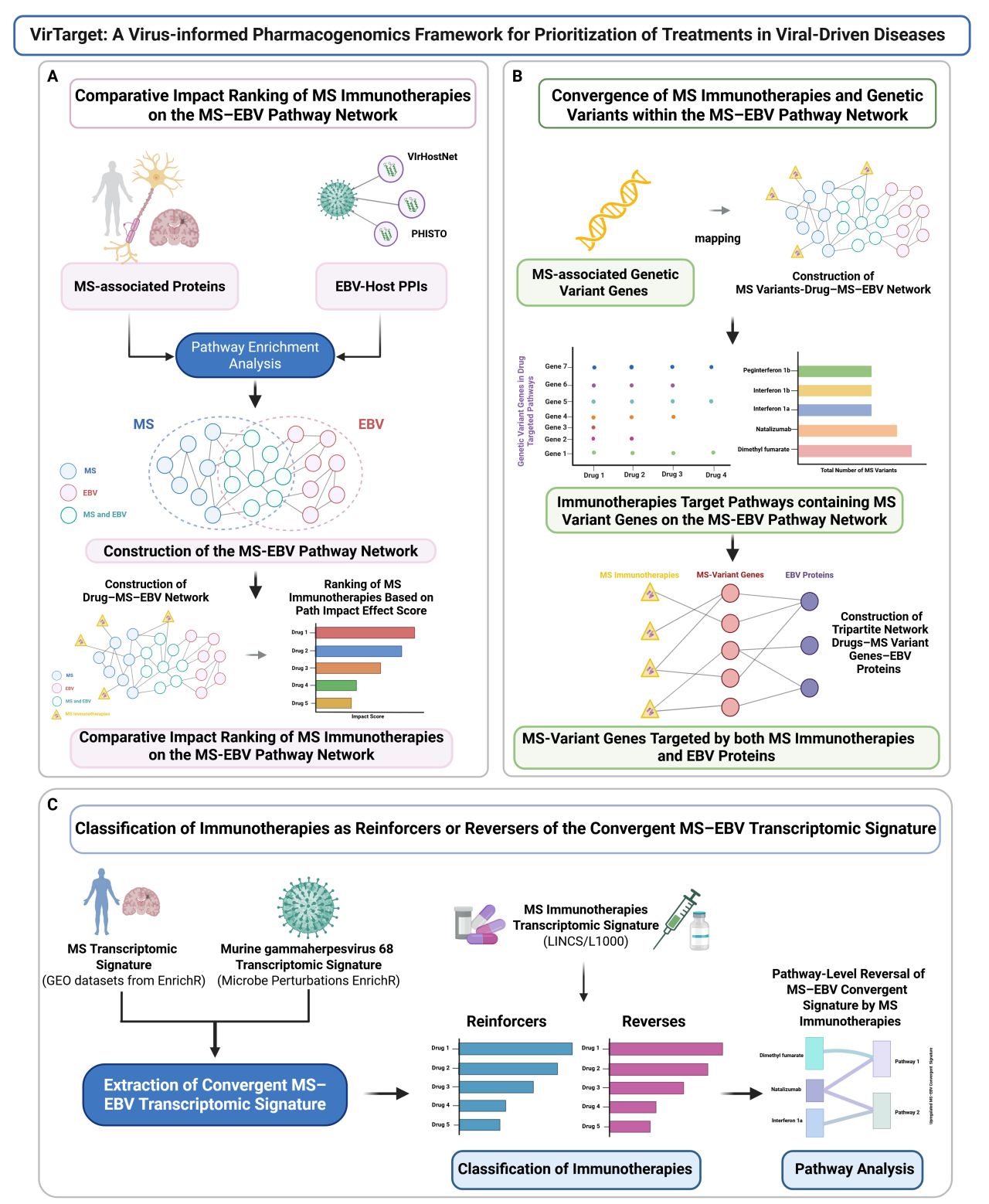
The AI & Systems Bioinformatics Unit of the Translational Neuropharmacology Lab has released a new preprint introducing VirTarget, the first virus-informed pharmacogenomics framework designed to systematically evaluate how multiple sclerosis (MS) immunotherapies interact with Epstein-Barr virus (EBV)-driven pathogenesis.
While EBV is increasingly recognized as a central driver of MS, current immunotherapies are prescribed without considering their effects on viral-host interactions or how they modulate EBV-related genetic risk factors. To address this critical gap, VirTarget integrates three complementary analytical layers: EBV-host interactomics mapping viral-host protein interactions within MS-relevant pathways, host genetic susceptibility linking MS risk variants to EBV-targeted therapy-modulated networks, and transcriptomic directionality analysis that classifies therapies as reinforcers or reversers of the MS-EBV signature.
Our systematic evaluation of approved MS immunotherapies revealed substantial heterogeneity in their engagement with the MS-EBV network. Dimethyl fumarate showed the strongest and broadest network engagement, followed by natalizumab and interferons, while anti-CD20 antibodies and S1P modulators exhibited weaker effects on EBV-related pathways. Notably, therapies converged on critical MS risk genes including HLA-DRB1, IL7R, IL2RA, CD40, and TYK2, which are directly targeted by EBV proteins within therapy-modulated pathways. Multiple therapies reversed EBV-driven dysregulation of cytokine and innate immune pathways, suggesting their benefits extend to counteracting viral-mediated immune modulation.
VirTarget establishes a foundation for precision treatment strategies in MS, where therapeutic selection can be informed by patient viral serostatus, individual genetic susceptibility profiles, and system-level transcriptomic responses. This systems-level approach represents a paradigm shift toward virus-informed precision medicine in MS and other EBV-associated autoimmune diseases.
Read the full pre-print: Altas B, Georgiou P, Onisiforou M, Zanos P, Onisiforou A. VirTarget: Virus-Informed Pharmacogenomics Framework Identifies Immunotherapies That Mitigate Epstein-Barr Virus-Driven Dysregulation in Multiple Sclerosis. bioRxiv (2025). doi: 10.1101/2025.11.03.686445
This work was led by Dr. Anna Onisiforou, head of the AI & Systems Bioinformatics Unit.
New preprint: VirTrack framework reveals stage-specific EBV pathogenesis in Multiple Sclerosis.
We’re excited to share our latest preprint, “VirTrack: A Framework for Inferring Viral Influence on Disease-Associated Transcriptomes — Clinical Type-Specific Epstein–Barr Virus Pathogenesis in Multiple Sclerosis,” now available on bioRxiv. This work, led by Dr. Anna Onisiforou, Head of our AI & Systems Bioinformatics Unit, represents a major advance in understanding how Epstein–Barr virus (EBV) contributes to Multiple Sclerosis across different disease stages. While the link between EBV and MS has been established during the last decade, how the virus influences the disease differently in early versus progressive forms has remained a critical unanswered question.
To address this, our team developed VirTrack, a novel computational framework that integrates experimentally validated EBV–host protein interactions with clinical type–specific gene expression data from patients with Clinically Isolated Syndrome, Relapsing Remitting MS, Secondary Progressive MS, and Primary Progressive MS. Using machine learning and systems biology approaches, VirTrack revealed striking clinical type–dependent patterns of viral engagement. In early MS stages, EBV targeted 13–18% of dysregulated genes, driving B-cell processes, inflammatory responses similar to active infection, and suppression of antiviral defenses. Remarkably, progressive forms showed a mechanistic shift: Secondary Progressive MS exhibited suppression of vascular and cardiac repair pathways, while Primary Progressive MS was characterized by upregulation of cellular remodeling processes. The framework also identified a stable core of influential viral proteins, with EBNA-LP consistently emerging as the top-ranked player across disease types.
This research, funded by the Infectious Diseases Society of America, has important therapeutic implications. Our findings suggest that EBV-directed interventions may be most effective in early disease stages when viral influence on immune dysregulation is strongest, while also revealing potential connections between EBV activity and vascular complications in progressive MS. Beyond MS, VirTrack offers a generalizable systems-level tool for investigating viral contributions to other complex human diseases, opening new avenues for understanding host-pathogen interactions across the disease spectrum.
To read the full article, please visit: https://doi.org/10.1101/2025.10.05.680499
The Translational Neuropharmacology Lab presents on novel therapies for brain disorders.

Dr. Panos Zanos recently delivered a presentation on novel pharmacological therapies for brain diseases at the Ierokipio University in Paphos, Cyprus. The Ierokipio University is a lifelong learning initiative that brings scientific knowledge to the general public, fostering dialogue between researchers and community members on important health and medical topics.
The presentation provided an overview of the Translational Neuropharmacology Lab’s research on innovative approaches to treating various brain disorders, with particular focus on depression, substance use disorders, and other neuropsychiatric conditions. Dr. Zanos discussed how emerging pharmacotherapies, including ketamine and other novel compounds, are being investigated for their potential to address treatment-resistant conditions where conventional medications have proven inadequate.
A significant portion of the talk focused on the PROUD (Preventing Relapse in Opioid Use Disorder) study—Cyprus’s first drug-intervention clinical trial—which investigates ketamine’s potential to treat comorbid depression in individuals recovering from opioid addiction. The presentation also covered the broader landscape of ketamine research, including its rapid-acting antidepressant properties and potential applications across various psychiatric conditions.
Dr. Zanos explained the neurobiological mechanisms through which these novel pharmacotherapies may exert their therapeutic effects, including their actions on neurotransmitter systems, synaptic plasticity, and brain circuitry. The presentation emphasized the importance of rigorous preclinical and clinical research in translating laboratory discoveries into evidence-based treatments that can improve patients’ lives.
Brain disorders, including depression, anxiety, substance use disorders, and neurodegenerative diseases, represent major public health challenges affecting millions of people worldwide. Traditional treatments, while beneficial for many, leave significant gaps in care, particularly for individuals with treatment-resistant conditions. The lab’s research aims to identify and validate novel therapeutic targets and compounds that can address these unmet medical needs.
Public engagement activities like this presentation are essential for bridging the gap between scientific research and community understanding. By sharing our work with the broader public, we aim to increase awareness about brain disorders, reduce stigma surrounding mental health and addiction, and foster informed public discourse about the development of innovative treatments. The interactive format allowed for valuable questions and discussions, highlighting the community’s interest in understanding both the challenges of treating brain disorders and the promise of emerging therapies.
We are grateful to the Ierokipio University in Paphos for providing this platform to share our research with the community and look forward to continued engagement with the public as our research program advances.
Dr. Zanos was invited and presented on antidepressant mechanisms of action of ketamine and other rapid-acting antidepressants at the 6th German-Cypriot Psychotherapy Conference.

Dr. Panos Zanos delivered an invited presentation at the 6th German-Cypriot Conference Psychotherapy Dialogues, titled “Neurobiology of Depression: Traditional & Rapid-acting Pharmacotherapies.”
The presentation addressed a critical challenge in psychiatry: conventional antidepressants require three to four weeks to produce therapeutic effects, with approximately thirty percent of patients failing to respond. Dr. Zanos explored the neurobiological foundations of depression, including HPA axis dysfunction, the monoaminergic theory and its limitations, and how chronic stress affects brain plasticity through reduced neurogenesis and synaptic loss in the hippocampus and prefrontal cortex.
A major focus was on rapid-acting antidepressants, particularly ketamine, which produces significant antidepressant effects within hours rather than weeks. Dr. Zanos discussed four mechanistic hypotheses for ketamine’s rapid action involving NMDA receptor blockade, BDNF release, and mTORC1 pathway activation, as well as the possible involvement of ketamine’s hydroxynorketamine metabolites. He also presented exciting clinical developments, including ongoing Phase II studies of hydroxynorketamine—a ketamine metabolite with potentially fewer side effects—led by Dr. Carlos Zarate at NIMH.
Featured in Nature Mental Health: Advancing ketamine research and brain pathology treatments.
We are happy to share that Dr. Zanos, the Director of the Translational Neuropharmacology Lab, was recently featured in a Q&A article published by Nature Mental Health. The discussion explores our laboratory’s pioneering work on ketamine and its metabolites as novel treatments for brain pathologies, with particular emphasis on depression and substance use disorders.
The conversation delves into the unique ability of ketamine to rapidly alleviate symptoms in individuals with treatment-resistant depression, a condition where traditional antidepressants often fail or take weeks to become effective. Our research has focused extensively on (2R,6R)-hydroxynorketamine, a bioactive metabolite of ketamine that shows promise in preclinical studies and may offer therapeutic benefits with reduced side effects compared to ketamine itself. This compound has now advanced to phase 2 clinical trials for treatment-resistant depression and neuropathic pain, with phase 1 results demonstrating excellent tolerability in humans.
The article also highlights our groundbreaking work in Cyprus, where we are conducting the country’s first drug-interventional clinical trial. This phase 2, double-blind, placebo-controlled study is evaluating ketamine’s effectiveness in reversing comorbid affective disorders and maintaining opioid abstinence. This initiative represents a significant step forward in addressing opioid-use disorders, a critical public health concern both locally and globally.
Beyond our specific research projects, the discussion touches on important methodological considerations in neuroscience research, including the role of animal models in understanding human brain disorders and the need for more sophisticated translational approaches. Dr. Zanos also spoke about his role as founding member and president of the Cyprus Neuroscience Society, which works to advance neuroscience research in Cyprus through knowledge sharing, training opportunities, and international collaboration.
Looking toward the future, Dr. Zanos expresses optimism about the potential of ketamine metabolites to address treatment-resistant depression and substance-use disorders, as well as the broader field of psychedelics as pharmacotherapies for brain disorders. With growing evidence demonstrating effectiveness and collaborative initiatives like the PSY-NET COST Action involving researchers across many European countries, there is strong momentum toward reshaping mental health care and offering hope to millions of patients worldwide.
This feature in Nature Mental Health underscores the impact and reach of the work being conducted at the Translational Neuropharmacology Lab, and we are honored to contribute to the global conversation on advancing treatments for brain pathologies.
To read the full Q&A article visit Nature Mental Health: https://www.nature.com/articles/s44220-025-00511-6.
Researchers from the Zanos lab attend the European Researchers’ Night 2025.

We are pleased to share that the Translational Neuropharmacology Lab participated in the European Researchers’ Night (ERN) 2025, held on September 26, 2025, at the Cyprus State Fair in Nicosia. This annual event, organized by the Research and Innovation Foundation, brings research and innovation closer to the public through interactive demonstrations and activities.
Our team had the opportunity to present our research methodologies to students and members of the general public. During our demonstration, we showcased EEG technology used in our human studies, allowing visitors to see firsthand how we monitor brain activity and study neural processes. We also explained our research approaches in translational neuropharmacology, helping bridge the gap between laboratory work and clinical applications.
The European Researchers’ Night featured over 70 interactive activities designed to make science accessible to audiences of all ages. Our participation allowed us to engage with the community, answer questions about neuroscience research, and share insights into the work being conducted in Cyprus. The event provided a valuable platform to demonstrate how our research contributes to advancing understanding of brain function and developing new therapeutic approaches.
We thank everyone who visited our demonstration and showed interest in our work. Events like ERN play an important role in connecting the scientific community with the public and fostering appreciation for research and innovation. We look forward to continuing our outreach efforts and participating in future events that promote scientific education and awareness.
For more information about our research and activities, visit www.zanoslab.com. To learn more about the European Researchers’ Night, visit www.erncyprus.com.
Dr. Zanos has been recognized among world’s top 2% of scientists in the 2024 Stanford University database
We are pleased to announce that Dr. Zanos, Director of the Translational Neuropharmacology Lab, has been included in the Stanford University database of the world’s top 2% scientists, published by Elsevier. This prestigious recognition places Dr. Zanos among an elite group of researchers globally and reflects the significant impact of our laboratory’s research contributions to the field of neuropharmacology.
The Stanford database represents a comprehensive analysis of scientific impact across all academic disciplines worldwide. Scientists are evaluated based on citation impact, research contributions as lead author, field-normalized metrics that account for disciplinary differences, and long-term research influence. Being listed in this database demonstrates that the work conducted in our laboratory has made meaningful contributions to advancing our understanding of translational neuropharmacology and has influenced the broader scientific community.
This achievement represents not only Dr. Zanos’ individual research excellence but also the collective efforts of our entire laboratory team, including current and former graduate students, postdoctoral researchers, and collaborators who have contributed to our research program over the years. The recognition underscores the laboratory’s commitment to conducting high-impact research that bridges basic neuroscience with clinical applications.
For more information, visit: https://elsevier.digitalcommonsdata.com/datasets/btchxktzyw/8
Zanos Lab research featured in the Cypriot “Politis” Newspaper

Our Master’s student and registered pharmacist, Georgios Kousathanas, was recently featured in the Cypriot “Politis” newspaper discussing one of our research topics on ketamine as an antidepressant treatment. The article, titled “Ketamine as a Model Drug for Rapid Antidepressant Action”, covers a part of the work being conducted in our laboratory on ketamine’s rapid-acting therapeutic properties. In this article, Georgios’ discusses how very low doses of ketamine offer faster, within hours, antidepressant effects compared to traditional medications, which typically take weeks to show results. This work contributes to understanding potential new treatment approaches for patients with treatment-resistant depression.
The newspaper coverage brings attention to this area of research and its potential clinical applications. As both a pharmacist and researcher, Georgios provides valuable insights into ketamine’s therapeutic mechanisms. We’re pleased to see our lab’s work on novel therapeutic approaches in neuroscience and mental health treatment gaining media attention.
Dr. Eleftheria Charalambous presents novel research findings on microbiome and brain health.
We’re happy to share that our postdoctoral researcher Dr. Eleftheria Charalambous recently presented her latest findings at the World Federation of Societies of Biological Psychiatry (WFSBP) 2025 Congress. Her innovative research focuses on gut microbiome-wide association studies that identify specific viruses linked to mental health and brain aging.
Dr. Charalambous’s work represents a significant advancement in understanding the gut-brain axis, specifically examining how viral components of the gut microbiome may influence neurological and psychiatric outcomes. This cutting-edge research opens new avenues for understanding the complex relationships between our microbial communities and brain health.
These findings from are currently under review for publication. We look forward to sharing more details about this important research as it progresses through the peer-review process.
Our lab members were selected and participated in a leadership training workshop through European COST actions.

We are pleased to announce that Drs. Zanos and Apostolakis from the Translational Neuropharmacology Lab have been successfully selected and completed the “COST Academy – Leadership training workshop” for early-career investigators. This specialized training workshop was designed for young researchers to develop leadership skills for positions within COST consortia and broader research environments.
Our laboratory’s participation reflects our active involvement in important COST Actions. Dr. Zanos represents our lab in the “Psychedelic renaissance: turn on, tune in and drop in” COST Action (CA24130), which aims to advance psychedelic research by networking academic institutions with the pharmaceutical industry to develop new treatments for depression, anxiety, and addiction. Dr. Apostolakis contributes to the “EEG101: Fundamentals of Open & Rigorous EEG Science” COST Action (CA24148), which focuses on standardizing EEG research practices to improve reproducibility and enable better clinical applications.
This leadership training enhances our laboratory’s capacity to drive international collaboration, lead research initiatives, and advance open science practices. The skills and networks developed through this program will directly benefit our ongoing research in translational neuropharmacology and our contributions to the international neuroscience community.
Infrastructure grant awarded to the Zanos Lab: TRACER Research Center.
The Zanos Lab is proud to announce the award of €500,000 from the Research Promotion Foundation of Cyprus to establish the Translational Neuroscience and Behavioural Pharmacology Research Center (TRACER). This infrastructure grant will establish Cyprus’s first comprehensive neuropharmacology and systems neuroscience research facility, positioning our laboratory and the University of Cyprus at the forefront of translational brain research. TRACER represents a paradigm shift in our capacity to investigate neural mechanisms underlying psychiatric and neurological disorders.
The TRACER center will integrate advanced technologies including:
- Multi-fiber photometry systems for real-time monitoring of neural circuit activity across multiple brain regions in rodents.
- Wireless miniscope calcium imaging for longitudinal monitoring of neural activity in freely behaving animals.
- Advanced electrophysiology platforms with dual-channel patch-clamp and field potential recording capabilities
- Behavioral pharmacology operant conditioning chambers for investigating addiction and reward mechanisms
- Integration with existing infrastructure including EEG/EMG systems and comprehensive behavioral testing batteries
TRACER will enable investigation of previously inaccessible research questions in Cyprus by providing real-time, multi-modal neural monitoring during complex behavioral paradigms. This approach is essential for understanding neuropsychiatric disorders where symptoms emerge from dysfunctional interactions across multiple neural circuits.
Building on our laboratory’s track record of breakthrough discoveries—including the identification of ketamine metabolites now in Phase II clinical trials for depression treatment—TRACER will accelerate therapeutic discovery and translation from preclinical findings to clinical applications.
This grant was awarded under the Research Promotion Foundation’s “RESTART 2016-2020” program, Pillar I (Smart Growth), specifically targeting research infrastructure development. The award reflects the exceptional scientific merit and strategic importance of establishing advanced neuroscience capabilities in Cyprus.
Dr. Andria Michael brings Neuroimmunology to young minds

We’re excited to share that our postdoctoral researcher Dr. Andria Michael has authored an insightful article in Neolaia, Cyprus’s youth newspaper, explaining the fascinating connection between the immune system, neuroscience and depression.
In her piece titled “How Does the Immune System Connect with Depression?”, Dr. Michael breaks down complex neurobiological concepts for young readers, exploring how immune dysfunction can contribute to depressive disorders. The article highlights cutting-edge research in neuropsychiatry, including the role of neuroinflammation and stress responses in mental health. This is an important piece of work since it (i) bridges the gap between advanced neuroscience research and public understanding; (ii) introduces young Cypriots to the exciting field of neuropharmacology and immunology; (iii) demonstrates how immune-brain interactions are reshaping our approach to treating depression
Dr. Michael’s commitment to science communication reflects our lab’s dedication to not only advancing knowledge but also sharing it with the broader community. By making complex research accessible to young minds, we’re inspiring the next generation of neuroscientists.
Exploring Misophonia via identifying innovative biomarkers.

Our Ph.D. student, Ms. Despina Melanthiou presented her research project on identifying novel biomarkers for misophonia. Her work, that is being conducted at the University of Cyprus, explores the use of electroencephalography (EEG), heart rate variability (HRV), and other neuropsychophysiological markers, alongside biological markers from blood samples, to better understand this complex sensory processing disorder. Misophonia, characterized by intense emotional reactions to specific sounds, affects countless individuals worldwide, and Despina’s research is a vital step toward unraveling its underlying mechanisms.
Despina’s study will be capturing brain activity patterns, integrating HRV, a well-established measure of autonomic nervous system function, providing insights into the neural and physiological correlates of misophonia. Additionally, her analysis of blood-based biomarkers aims to identify molecular signatures that could serve as objective indicators of misophonia, addressing a critical gap in current diagnostic approaches. The significance of Despina’s work lies in its innovative combination of neuropsychophysiological and biological markers, a relatively underexplored area in misophonia research.
New preprint: Transcriptomic insights into (2R,6R)-hydroxynorketamine’s possible therapeutic effects for Opioid Use Disorder
We are excited to share our latest research led by Dr. Anna Onisiforou with contributions from Dr. Andria Michael and Mr. Morfeas Koumas now available as a preprint on bioRxiv, titled “Transcriptomic mapping of the ventral hippocampus reveals (2R,6R)-hydroxynorketamine as a potential therapeutic agent for opioid abstinence.” This study investigates the therapeutic potential of (2R,6R)-hydroxynorketamine (HNK), a metabolite of ketamine, in addressing the emotional and molecular deficits caused by opioid abstinence.
Our research utilized a three-week opioid abstinence model in male C57BL/6J mice to explore how (2R,6R)-HNK mitigates the effects of chronic morphine exposure. We found that (2R,6R)-HNK restored sucrose and social preference in morphine-abstinent mice, effectively reversing deficits in reward and social behavior. Through RNA sequencing of the ventral hippocampus, we identified 206 differentially expressed genes in morphine-abstinent mice compared to controls, with (2R,6R)-HNK reversing key molecular changes. Notably, machine learning analysis highlighted genes such as Il1rapl1 and Ctla2b as top predictors of treatment response, underscoring their potential as biomarkers.
The study also revealed that (2R,6R)-HNK’s effects are context-specific. While it induced transcriptional changes in opioid-naive mice, it only improved behavior in those with prior morphine exposure, demonstrating its targeted efficacy in pathological states like Opioid Use Disorder. With OUD affecting 27 million people globally and contributing to 480,000 deaths annually, these findings suggest (2R,6R)-HNK, that is currently under phase II clinical development, could be a promising intervention to reduce relapse risk by addressing persistent emotional and molecular changes during abstinence. However, some neuroimmune and behavioral pathways remained dysregulated even following treatment with HNK, indicating an intermediate recovery state that warrants further exploration with additional behavioral models.
Read the full preprint article here: https://www.biorxiv.org/content/10.1101/2025.06.04.657935v1
Dr. Apostolakis Shares Specialized Research Insights on Nicotine Addiction at European Psychology Congress Workshop
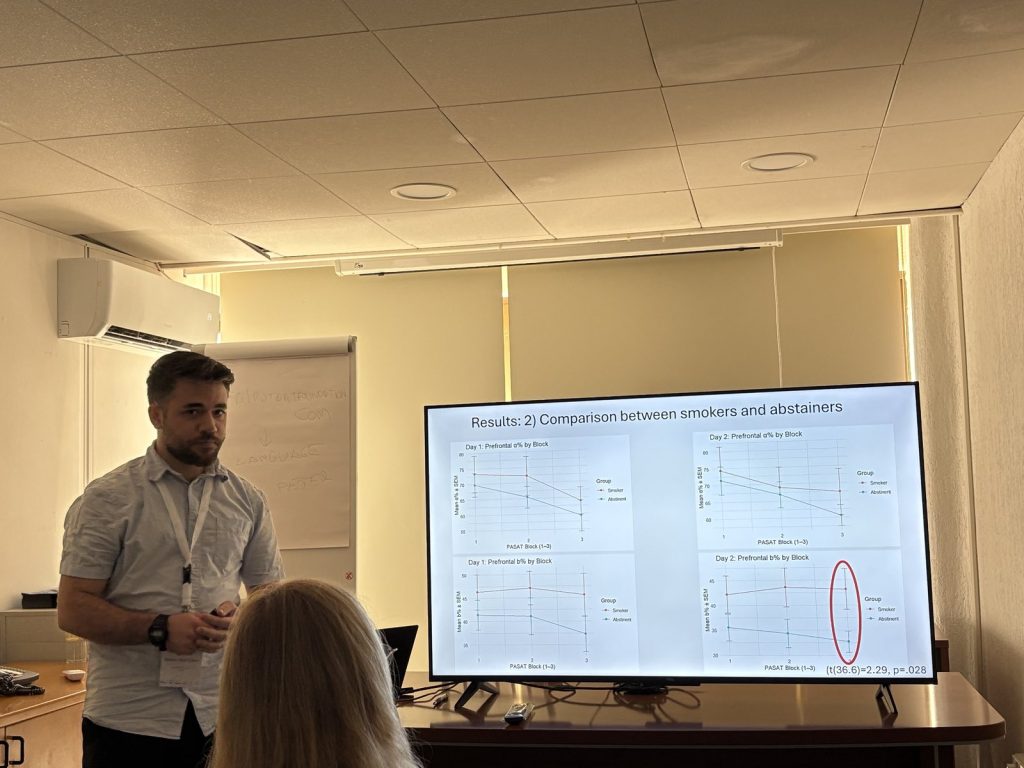
The Translational Neuropharmacology Lab is proud to announce that Dr. Apostolakis delivered a compelling presentation at the 19th European Congress of Psychology, held July 1-4, 2025, in Paphos, Cyprus. His research presentation, “Stress biomarkers and emotion regulation difficulties as predictive factors of vulnerability to nicotine addiction relapse,” highlighted groundbreaking findings in the field of smoking cessation science.
Understanding relapse mechanisms remains one of the most pressing challenges in nicotine addiction treatment, with stress-induced relapse representing a primary obstacle to successful long-term cessation. Dr. Apostolakis’s investigation explored the complex relationship between withdrawal-induced stress sensitivity and individual capacity for emotional self-regulation in predicting treatment outcomes.
The research involved a carefully designed two-day experimental study examining adults aged 18-65 during their smoking cessation attempts. Using the Paced Auditory Serial Addition Test (PASAT) as a stress induction paradigm, the team measured participants’ responses both during normal smoking conditions and following 24 hours of nicotine withdrawal. Electroencephalography recordings captured neural stress responses across both conditions, while the Difficulties in Emotion Regulation Scale (DERS) assessed individual differences in emotional coping strategies. Three-month follow-up assessments tracked long-term cessation success.
The study’s central hypothesis predicted that withdrawal would trigger measurable changes in cortical activity—specifically reduced alpha wave activity and elevated beta wave activity in prefrontal brain regions—reflecting heightened stress vulnerability. Additionally, the research tested whether individual differences in emotion regulation capacity would amplify these withdrawal-related stress responses, ultimately predicting relapse likelihood.
This innovative research represents a significant advancement in understanding the neurobiological and psychological factors that contribute to smoking relapse. The findings have important implications for developing personalized treatment approaches that address individual vulnerability profiles. The presentation generated considerable interest among international researchers and underscores the Translational Neuropharmacology Lab’s commitment to addressing critical public health challenges through rigorous scientific investigation.
Dr. Zanos receives the 2025 Research Excellence Award from the University of Cyprus.

We are excited to announce that Dr. Zanos, the Director of the Translational Neuropharmacology Laboratory, has been honored with the prestigious 2025 Research Excellence Award from the University of Cyprus. The award, presented by the Minister of Education of Cyprus on June 25, 2025, recognizes Dr. Zanos’ and our lab’s groundbreaking contributions to neuroscience and the transformative impact on research infrastructure in Cyprus.
Dr. Zanos’ pioneering work focuses on the discovery of novel rapid-acting antidepressants, with significant findings published in top-tier journals such as Nature, PNAS, Nature Neuroscience, e.t.c.. His research has led to a successful Phase I clinical trial for a new antidepressant compound in collaboration with the National Institute of Mental Health (USA), with Phase II trials now underway to evaluate its efficacy for severe depression. Additionally, Dr. Zanos is leading three innovative clinical trials in Cyprus, including the nation’s first interventional drug trial to prevent opioid relapse and studies exploring the neurobiological mechanisms of nicotine addiction and smoking cessation.
Despite being an early-career investigator, he has published over 75 articles in high-impact journals, secured patents in multiple countries, and obtained over €2.2 million in competitive funding during his first 4 years of independent position.
This award highlights Dr. Zanos’ commitment to translating cutting-edge research into real-world clinical applications, elevating Cyprus’ standing in the global scientific community.
We extend our congratulations to Dr. Zanos and the entire lab team for their dedication to advancing neuroscience through innovative research.
Dr. Zanos meets Prof. George Paxinos at the Mediterranean Neuroscience Society 2025 conference

We’re excited to share a special moment from the 10th Mediterranean Neuroscience Society Conference 2025 in Crete, Greece. Our lab director had the incredible opportunity to meet Professor George Paxinos AO, one of the most influential figures in modern neuroscience.
Professor Paxinos is universally recognized as “the man who mapped the brain.” He’s the author of “The Rat Brain in Stereotaxic Coordinates,” which for three decades was the third most cited book in science with over 73,000 citations. This groundbreaking atlas revolutionized neuroscience by providing the first reliable three-dimensional framework for brain studies – essentially the GPS that every neuroscientist relies on. With 114,118 total citations and 58 published books, he has identified 94 nuclei in rat and human brains, making him one of the most influential neuroscientists of our time.
This meeting is particularly meaningful because Professor Paxinos’s work directly enables what we do in our translational neuropharmacology lab. His brain atlases serve as essential navigational tools for our drug discovery work, helping us understand precise anatomical targets and ensure accuracy when targeting specific brain regions for therapeutic development. Most scientists working on neurologic or psychiatric diseases rely on his maps and concepts.
Dr. Zanos chairs ketamine research symposium at the Mediterranean Neuroscience Society conference 2025
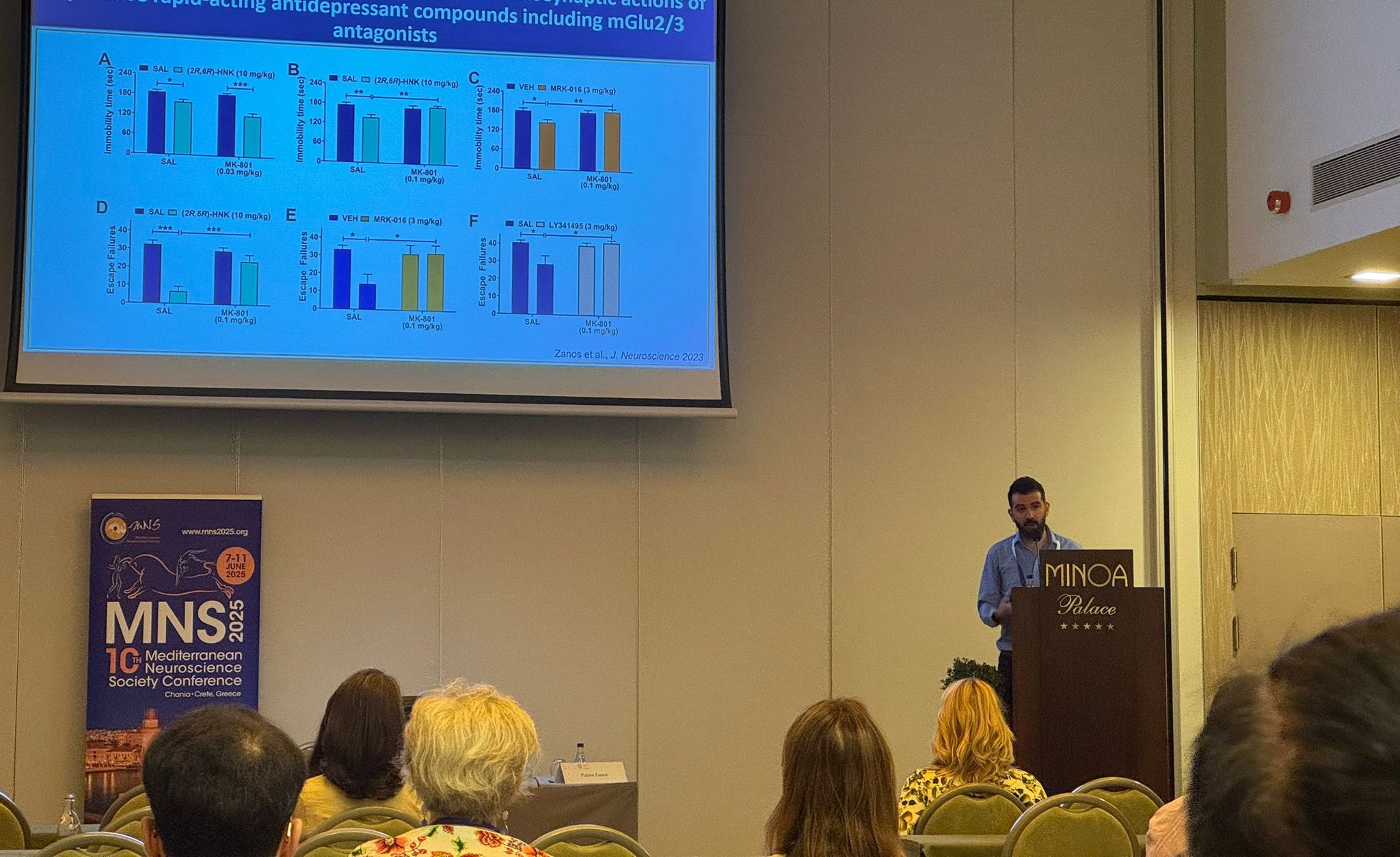
Dr. Panos Zanos represented our lab at the Mediterranean Neuroscience Society Conference in Crete, where he chaired a symposium on ketamine’s rapid antidepressant mechanisms. The symposium, titled “Unraveling ketamine’s rapid antidepressant mechanisms: From neural circuits to clinical implications,” brought together leading researchers from around the world to discuss cutting-edge findings on how ketamine works as an antidepressant.
Dr. Zanos presented our lab’s latest research on NMDA receptor activity and its role in ketamine’s rapid antidepressant effects. His presentation focused on how canonical NMDAR-dependent long-term potentiation is required for ketamine’s therapeutic efficacy, revealing an inverted U-shaped dose-response relationship that challenges simple NMDAR antagonism theories. The work demonstrates that excessive NMDA receptor inhibition may actually impede antidepressant properties, suggesting ketamine’s mechanisms are more complex than previously thought.
The symposium featured three other distinguished speakers who presented complementary research on corticotropin-releasing factor mechanisms, multi-omic approaches to understanding stress and ketamine interactions, and the complex relationship between ketamine and dopamine neurotransmission. Together, these presentations provided a comprehensive overview of the latest advances in understanding ketamine’s complex mechanisms of action, from molecular interactions to circuit-level effects.
Dr. Michael presents new research on the potential efficacy of ketamine’s metabolite hydroxynorketamine for opioid addiction
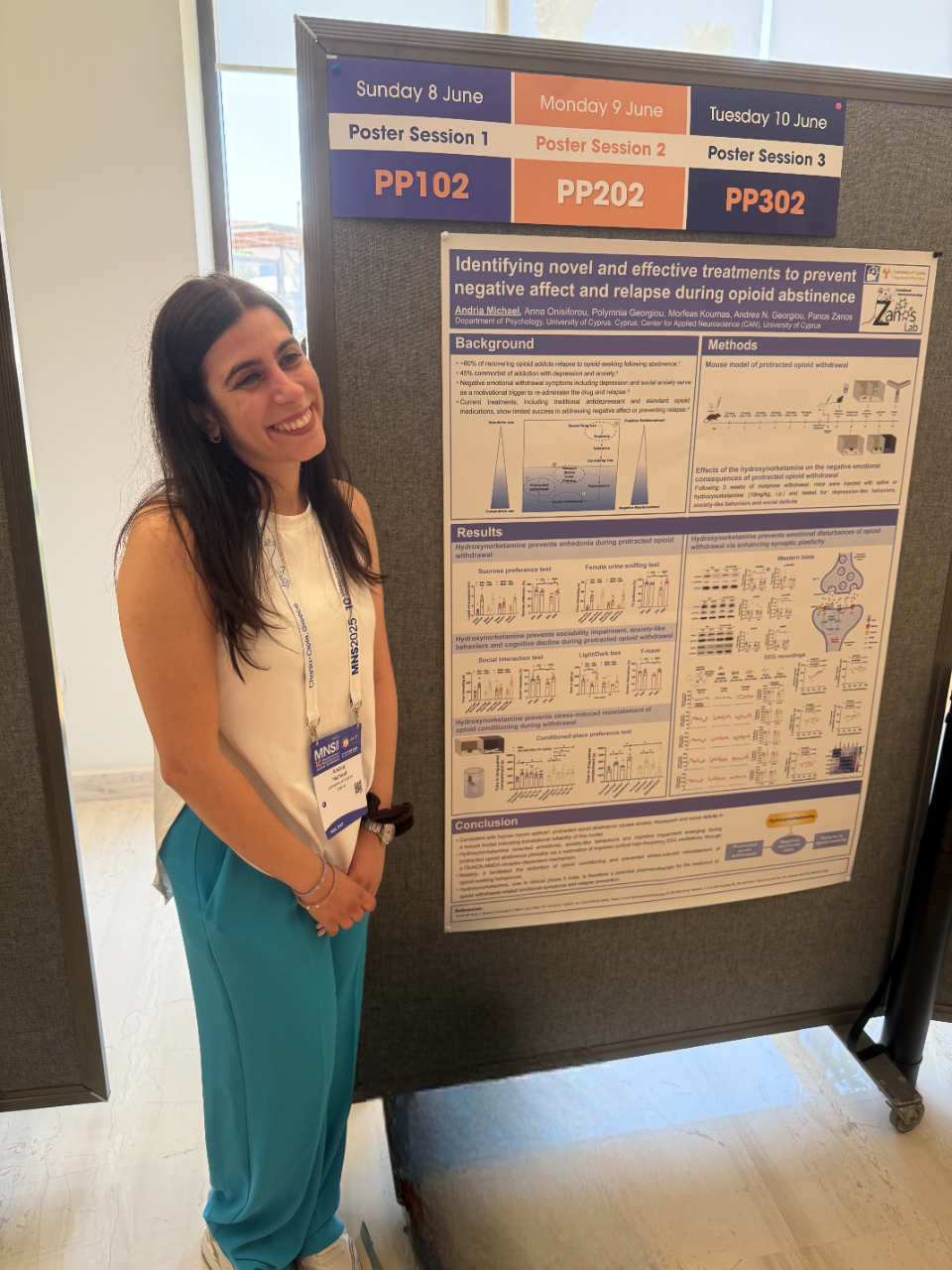
Dr. Michael (Marie Curie fellow from our lab) presented new research at the Mediterranean Neuroscience Society Conference in Crete, showcasing our latest findings on how a ketamine metabolite could help treat opioid addiction.
Her poster focused on (2R,6R)-hydroxynorketamine (HNK), a ketamine metabolite, and its effects on opioid addiction-related behaviors in mice. The research tackled one of the biggest challenges in treating opioid use disorder: the negative emotional states and high relapse rates during abstinence that current treatments struggle to address.
The study demonstrated that HNK could reverse conditioning to morphine in stress-susceptible mice, prevent negative withdrawal responses, and alleviate acute withdrawal symptoms. HNK also helped reverse anhedonia, anxiety, and cognitive problems during long-term abstinence by restoring impaired brain wave patterns through NMDA receptor mechanisms. Additionally, it facilitated faster extinction of opioid conditioning, prevented stress-induced relapse, and reduced morphine consumption in previously exposed mice.
What makes this research particularly promising is that HNK is already in Phase II clinical trials, meaning these findings could lead to new treatments faster than developing entirely new drugs. Given the ongoing opioid crisis, repurposing existing medications could be crucial for people struggling with addiction.
This research represents exactly what our translational neuropharmacology lab is about – taking fundamental brain discoveries and turning them into real solutions for those who need them most.
Dr. Zanos discusses social media’s impact on the brain at Pan-Cypriot Festival of Youth and Students event

Dr. Panos Zanos delivered an engaging discussion on “Social Media & Brain” as part of the 37th Pan-Cypriot Festival of Youth and Students. The talk explored the fascinating intersection between our digital habits and neurological processes, including insights from current research on how social media platforms influence brain function, potentially covering topics such as dopamine reward pathways, attention mechanisms, and the neurobiological basis of social media engagement. This timely discussion addressed one of the most pressing questions of our digital age: how does our constant connectivity with social media platforms reshape our brains?
This talk represents our lab’s ongoing commitment to public engagement and science communication, bringing neuroscience research to the broader community.
Dr. Polymnia Louka’s inspiring talk at the European University Cyprus annual conference

The Zanos Lab is delighted to share that Dr. Polymnia Louka, our Marie Skłodowska-Curie Fellow, delivered an engaging talk at the European University Cyprus (EUC) Annual Conference. Invited to address students and faculty, Dr. Louka discussed her journey to securing the prestigious and competitive Marie Curie Fellowship, as well as her innovative research exploring the effects of ketamine and other putative fast-acting antidepressants on depression-related bone health issues.
Her presentation highlighted the dedication and interdisciplinary approach that led to her success, inspiring students with a real-world example of perseverance in scientific research. Her talk also introduced her research project, which investigates how major depressive disorder contributes to bone health deterioration, such as increased risks of osteoporosis and fractures. Dr. Louka is studying whether rapid-acting antidepressants, specifically ketamine and its metabolites, can mitigate these bone impairments. She also explores the potential of combining ketamine with other agents to enhance therapeutic outcomes.
The conference provided an ideal platform for Dr. Louka to connect with a diverse audience. Her presentation captivated students and faculty, leading to a lively discussion session. Her collaborative efforts with the University of Cyprus and the Royal Veterinary College underscored the value of interdisciplinary research, motivating attendees to consider innovative approaches to complex health challenges.
We congratulate Dr. Louka on her successful talk and look forward to sharing more updates on her research, which holds promise for improving care for patients with depression and related conditions.
For more information about Dr. Louka’s work or the Zanos Lab, please contact us.
Zanos Lab part of the newly-awarded European COST action “PSY-NET” (Psychedelic renaissance).

We are pleased to announce that the Zanos Lab at the University of Cyprus is an integral part of the newly awarded COST Action “PSY-NET: Psychedelic renaissance: turn on, tune in and drop in” (OC-2024-1-27517).
This collaborative network brings together 83 leading researchers from 21 European countries to advance psychedelic research across multiple disciplines. The PSY-NET initiative aims to address the current challenges in developing novel treatments for mental disorders through coordinated research on psychedelic compounds and their therapeutic applications.
Dr. Panos Zanos contributes his expertise in neurochemistry and neuropharmacology to this interdisciplinary effort. As a secondary proposer in the network, Dr. Zanos will particularly focus on elucidating the mechanisms of action underlying the rapid antidepressant effects of ketamine and other psychedelic compounds.
The PSY-NET COST Action will focus on several key areas including chemistry and biophysics for the development of novel drug candidates and delivery systems; preclinical research to understand the neurobiological mechanisms of psychedelics; clinical applications through designing and implementing trials for depression, anxiety, addiction, and existential distress; advances in neuroimaging by standardizing data collection and analysis methods; data sharing through creation of open access databases for research collaboration; and policy regulations by working toward regulatory frameworks that enable clinical use.
This collaborative initiative represents a significant step forward in addressing treatment-resistant mental health conditions. As part of the PSY-NET COST Action, the Zanos Lab will contribute to developing recommendations and guidelines for psychedelic use in therapy, identifying neurobiological biomarkers and predictors, and establishing a regulatory framework to enable future clinical applications.
The involvement of the Zanos Lab in PSY-NET aligns with our ongoing research on ketamine’s rapid-antidepressant mechanisms of action, recently presented at the University of Bern’s Neuroscience Lecture Series. We look forward to this opportunity to collaborate with European research leaders and contribute to this rapidly expanding field of neuroscience and mental health treatment.
Dr. Zanos presented groundbreaking ketamine research at University of Bern’s Neuroscience lecture series.
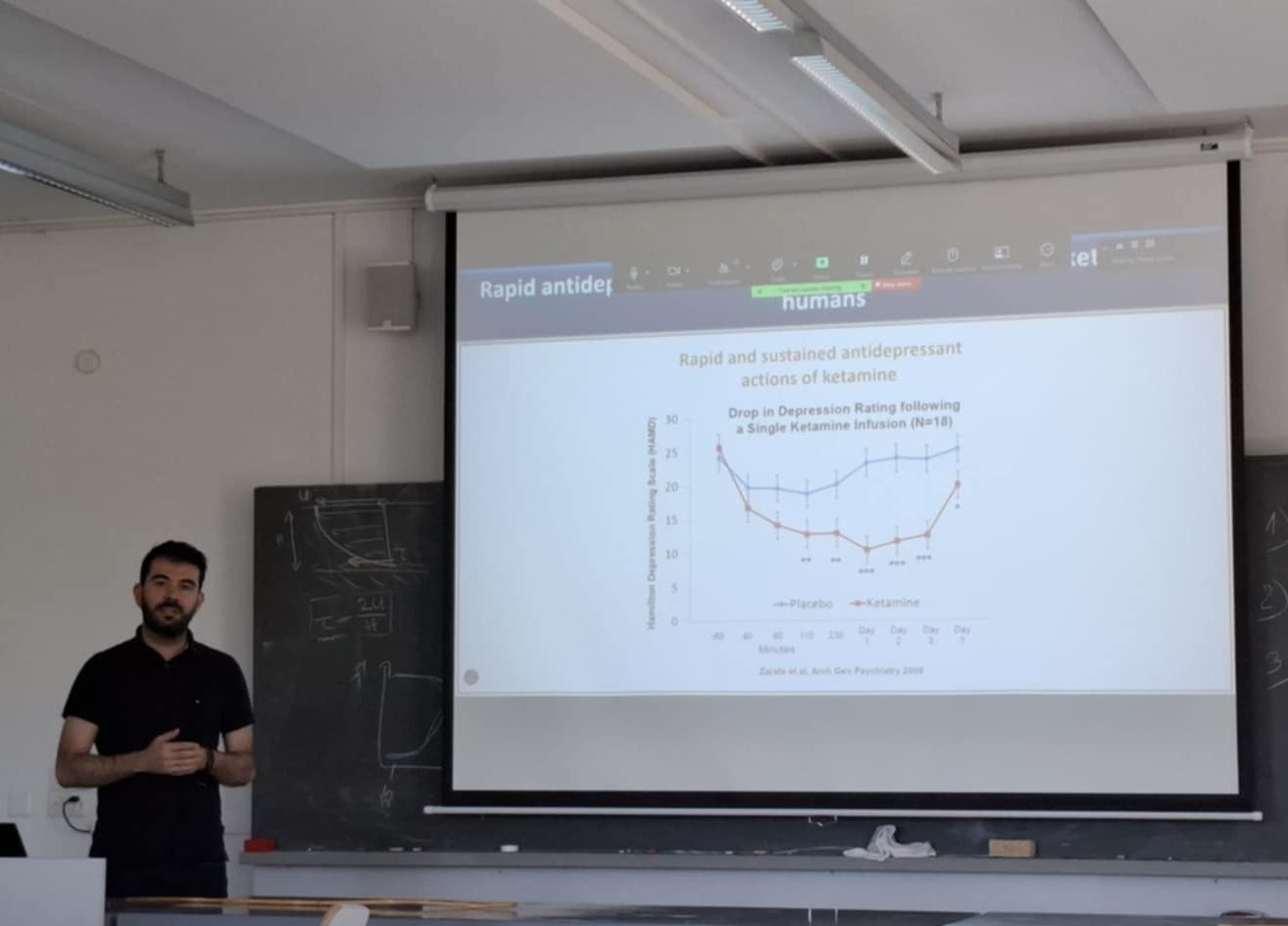
We are pleased to announce that Dr. Panos Zanos, Director of the Zanos Lab at the University of Cyprus, recently delivered a lecture as part of the prestigious Neuroscience Lecture Series (NLS) at the University of Bern, Switzerland.
Dr. Zanos presented the lab’s cutting-edge research on ketamine’s antidepressant properties, challenging the conventional understanding of its mechanism of action. While ketamine has revolutionized depression treatment with its ability to alleviate symptoms within hours (compared to weeks for traditional antidepressants), its clinical applications have been limited by side effects.
The presentation highlighted how Dr. Zanos’ research has revealed that NMDA receptor inhibition alone cannot fully explain ketamine’s sustained antidepressant effects. Instead, their work demonstrates the critical role of the (2R,6R)-hydroxynorketamine (HNK) metabolite, which exhibits antidepressant effects without ketamine’s side effects. Dr. Zanos discussed their discoveries regarding the paradoxical inverted U-shaped dose-response relationship and the importance of AMPA receptor activation in ketamine’s antidepressant actions.
This research establishes NMDAR activation as a common downstream effector for rapid-acting antidepressants and offers promising directions for developing next-generation antidepressants with improved efficacy and reduced side effects.
The NLS is a collaborative initiative between the Institute of Physiology and the Center for Experimental Neurology (ZEN), designed to foster high-quality scientific interactions with distinguished researchers. The invitation from Prof. Dr. Antoine Adamantidis, Prof. Dr. Stéphane Ciocchi, and PD. Dr. Carolina Gutierrez Herrera underscores the international recognition of our lab’s contributions to neuropharmacology and depression research.
Zanos Lab research decodes molecular pathways in Opioid Use Disorder and Depression at Hellenic Psychological Society conference

Dr. Zanos has presented new research of our lab at the Hellenic Psychological Society (ΕΛΨΕ) conference in Ioannina, Greece. The presentation, titled “Decoding common molecular pathways affected in opioid use disorder and depression,” explored the complex relationship between these frequently co-occurring conditions. This research was led by our lab members, Drs. Anna Onisiforou and Andrea Georgiou. Opioid use disorder (OUD) often coexists with major depressive disorder (MDD), but the exact underlying molecular and genetic mechanisms are unknown. Our research aimed to investigate the genetic and molecular mechanisms of association between OUD and MDD through Mendelian Randomization (MR), genetic correlation analysis, pathway enrichment analysis, and rodent models. The genetic correlation between OUD and MDD was calculated using linkage disequilibrium score regression (LDSC). MR analysis was performed to evaluate potential causal relationships, with sensitivity analyses to assess pleiotropy. Additionally, disease-associated protein enrichment analysis was conducted using the STRING application and ClueGO, with data from KEGG pathways to explore common molecular mechanisms. Rodent models with behavioral analyses were additionally utilized to identify brain-related biochemical mechanisms. The genetic correlation analysis revealed a significant positive correlation between OUD and MDD (rg = 0.3096, SE = 0.1475, p = 0.03). The MR analysis did not provide evidence for a causal relationship, with variance-weighted MR yielding an odds ratio (OR) of 0.99 (95% CI: 0.99–1.00, p = 0.26). Pathway enrichment analysis identified 69 common KEGG pathways between OUD and MDD, including key pathways such as calcium signaling and glutamatergic neurotransmission. Direct correlations were also found between cortical neuron activity and synaptic plasticity markers during opioid withdrawal with depressive behaviors in rodent models. Although no evidence for a direct causal relationship between OUD and MDD was observed through MR analysis, the genetic correlation and enrichment analysis highlight common molecular pathways between these disorders. These findings advance our understanding of comorbidity between OUD and MDD and suggest potential targets for therapeutic intervention. The research presented by Dr. Zanos represents a significant contribution to understanding the neurobiological basis of these commonly co-occurring disorders and may help develop more effective treatment strategies.
Zanos Lab Research on Nicotine Addiction Relapse Presented at Hellenic Psychological Society conference

The Zanos Lab is pleased to announce that Dr. Apostolakis has presented research at the Hellenic Psychological Society (ΕΛΨΕ) conference in Ioannina, Greece. The presentation, titled “Stress biomarkers and emotion regulation difficulties as predictive factors of vulnerability to nicotine addiction relapse,” addressed a critical challenge in smoking cessation efforts. Nicotine addiction is characterized by high relapse rates, often attributed to stress-related mechanisms during quit attempts. Dr. Apostolakis’s research examined how acute withdrawal enhances stress reactivity and how individual differences in stress management and smoking impulse control relate to cessation outcomes. The study aimed to identify physiological and psychological indicators that predict relapse, focusing specifically on stress reactivity and emotion regulation in a community sample of adults aged 18-65 who were attempting to quit smoking. The research employed a two-day experimental protocol during which the Paced Auditory Serial Addition Test (PASAT) was administered on day one and again after 24 hours of nicotine abstinence. Brain activity was evaluated using electroencephalography to record stress reactivity during both smoking satiation and 24-hour abstinence periods, while emotion regulation difficulties were assessed using the Difficulties in Emotion Regulation Scale (DERS). Smoking cessation maintenance was evaluated at the three-month mark. The researchers hypothesized that 24-hour abstinence would lead to decreased alpha wave power and increased beta wave power in prefrontal regions compared to the smoking satiation state, indicating increased stress response. Furthermore, the difference between satiation and abstinence states was expected to be moderated by difficulties in emotion regulation, with greater difficulties linked to increased stress reactivity and higher relapse rates. This important study aimed to enhance our understanding of relapse mechanisms and support the development of tailored interventions for nicotine addiction. The presentation was well-received by conference attendees and represents a significant contribution to addiction research from our lab.
Our postdoctoral fellow, Dr. Andria Michael, presents at YUFE4Postdocs workshop in University of Antwerp

We are pleased to announce that our postdoctoral researcher, Dr. Andria Michael, recently participated in the YUFE4Postdocs training workshop hosted at the University of Antwerp. The workshop focused on stakeholder interaction and open science practices, bringing together talented postdoctoral researchers from across the YUFE alliance.
During this spring workshop, Dr. Michael had the opportunity to present her ongoing research project to her peers and engage with potential stakeholders. This presentation allowed her to showcase her work, receive valuable feedback, and explore collaborative opportunities within the YUFE network
Dr. Michael’s participation in this workshop demonstrates the Zanos Lab’s commitment to fostering international collaborations and implementing open science principles in our research. Her engagement with stakeholders represents an important step in ensuring our research addresses real-world challenges and creates meaningful impact.
We look forward to seeing how the connections made and insights gained during this workshop will enhance Dr. Michael’s research and contribute to our lab’s ongoing projects.
For more information about YUFE4Postdocs and upcoming opportunities, visit the YUFE alliance website.
Zanos Lab postdoctoral researcher Dr. Polymnia Louka contributes expert article on depression and bone health

We are pleased to announce that Dr. Polymnia Louka of the Zanos Laboratory recently authored an informative article in a prominent Cypriot newspaper addressing the important relationship between depression and bone health.
In her article, Dr. Louka explains that depression is one of the most common psychiatric disorders in the modern world, affecting approximately 5% of the adult population. She discusses the established connection between depression and osteoporosis, highlighting how individuals with depression face increased risk of fractures and bone density loss, with women with major depressive disorder being particularly vulnerable.
Dr. Louka’s article describes how selective serotonin reuptake inhibitors (SSRIs) may influence bone mineral density, potentially increasing fracture risk by up to 40% in certain populations. This important public education piece emphasizes the need for healthcare providers to consider bone health when treating patients with depression.
Additionally, Dr. Louka discusses recent developments regarding ketamine as an innovative treatment for depression, particularly for patients who don’t respond to conventional therapies. The article suggests that addressing depression effectively might also benefit bone health, presenting a more holistic approach to patient care.
This publication represents our commitment to public education on the interactions between neurological conditions and physical health outcomes.
Dr. Louka’s researcher on ketamine and bone health is funded by a Marie Skłodowska-Curie co-fund scheme.
Zanos Lab awarded $100,000 IDSA foundation grant for Alzheimer’s Disease research
We are pleased to announce that Dr. Anna Onisiforou and Dr. Zanos have been awarded a $100,000 grant from the Infectious Diseases Society of America (IDSA) Foundation. The grant will support an innovative project entitled “Sex-Specific Mechanisms of HSV-1 and EBV in Alzheimer’s Disease Risk.”
This competitive funding was awarded through the 2025 Microbial Pathogenesis in Alzheimer’s Disease grant program, recognizing the potential impact and scientific merit of our research. The funded research aims to investigate the relationship between viral factors and Alzheimer’s disease development, with particular attention to sex differences in disease susceptibility. The project will explore how genetic variations and hormonal factors might interact with viral exposures to influence disease risk.
This work builds upon our previous IDSA Foundation-funded research, which has already yielded important insights into the complex relationship between microbial factors and neurodegenerative processes. This two-year project begins in May 2025 and findings from this research could contribute to our understanding of the biological factors that influence Alzheimer’s disease risk and potentially inform future therapeutic approaches.
Cyprus Neuroscience Society now officially established: A historic milestone for Cyprus’ neuroscience community led by Dr. Zanos
The Translational Neuropharmacology Lab is proud to announce that the Cyprus Neuroscience Society (C.N.S.), founded and presided over by our Principal Investigator Dr. Panos Zanos, has reached another significant milestone with the launch of its official website: cnscyprus.com.
This achievement builds upon Dr. Zanos’s vision to establish the first-ever neuroscience society in Cyprus (Registration Number: ΛΕΥ|Σ|0438), which he successfully established in 2024. As Founding President, Dr. Zanos continues to lead this pioneering organization, bringing together 20 founding members from various neuroscience disciplines across the island.
The new website represents a crucial advancement for neuroscience in Cyprus, creating a digital platform that connects researchers, clinicians, students, and enthusiasts under Dr. Zanos’s leadership. This initiative further demonstrates our lab’s commitment to positioning Cyprus as a hub for neuroscience excellence in the Eastern Mediterranean region.
Through his dual roles as Principal Investigator of our lab and President of the C.N.S., Dr. Zanos is uniquely positioned to foster collaborations that bridge laboratory research with broader scientific community engagement. The society, under his guidance, aims to advance neuroscience research, enhance education, raise public awareness, and represent Cyprus in the global neuroscience community.
The website launch is yet another example of how our lab, through Dr. Zanos’s leadership, continues to make significant contributions to the scientific landscape of Cyprus, creating infrastructure that will benefit generations of neuroscientists to come.
We invite everyone to visit cnscyprus.com to learn more about this groundbreaking initiative and the ongoing efforts of our lab’s Principal Investigator to elevate neuroscience research and education in Cyprus.
Dr. Zanos discusses innovative ketamine research at the American College of Greece (Deeree) faculty seminar

On Tuesday, April 8th, Dr. Zanos, Head of the Translational Neuropharmacology Lab, presented his groundbreaking research on ketamine’s antidepressant properties to faculty and students at Deree. The seminar, held in the Faculty Lounge, provided an intimate setting for in-depth discussion of novel therapeutic approaches to depression treatment.
Dr. Zanos’s presentation explored the molecular pathways through which ketamine and its metabolites produce rapid antidepressant effects. He detailed his lab’s investigations into (2R,6R)-hydroxynorketamine, highlighting its potential as a therapeutic agent that maintains antidepressant efficacy while eliminating ketamine’s dissociative side effects.
A significant portion of the talk focused on recent discoveries regarding the role of specific glutamate receptor subunits in mediating these effects, particularly the critical involvement of AMPA receptor activation and GluN2A-containing NMDA receptors in sustaining antidepressant responses.
The seminar concluded with a productive discussion about potential research collaborations between Dr. Zanos’s lab and Deree faculty. Several promising avenues for joint EU grant applications were identified, with particular interest in translational studies that could accelerate the development of next-generation rapid-acting antidepressants.
This visit represents an important step in fostering cross-institutional research initiatives aimed at addressing the urgent need for more effective treatments for depression and related disorders.
New study by the Bioinformatics Unit of the Zanos lab suggests Metformin may offer better Alzheimer’s prevention potential in Diabetes patients compared with Semaglutide
The Zanos lab has recently published a preprint study examining the comparative effectiveness of diabetes medications in potentially reducing Alzheimer’s disease risk. The research was led by our senior researchers Dr. Anna Onisiforou and Dr. Andrea Georgiou.
The study, titled “Metformin Shows Greater Potential Than Semaglutide in Reducing Alzheimer’s Risk in Diabetes Type II via Dual Actions: Tackling Disease Pathways and Environmental Herpesvirus Triggers,” employs an innovative multi-faceted approach combining network pharmacology, Mendelian Randomization analysis, and transcriptomic validation to evaluate 39 different diabetes treatments.
The team’s research reveals that metformin—one of the oldest and most widely prescribed diabetes medications—demonstrates superior potential for Alzheimer’s prevention compared to newer, high-profile medications like semaglutide (known commercially as Ozempic or Wegovy). This finding challenges assumptions that newer diabetes treatments would necessarily provide better neuroprotection.
We show that metformin works through multiple mechanisms:
- Activation of AMPK, insulin, and adipocytokine signaling pathways that regulate critical Alzheimer’s-related processes
- Potential indirect effects against herpesviruses, which are emerging as possible environmental contributors to Alzheimer’s disease
These results suggest we should reconsider how we approach Alzheimer’s prevention in diabetes patients. While semaglutide has received significant attention for its effects on weight loss, our data indicates it has minimal engagement with the core neurodegenerative pathways connecting diabetes and Alzheimer’s disease.
The study also identified several dual-action therapies with efficacy comparable to metformin, supporting the need for personalized approaches to treatment selection based on individual risk profiles.
This research represents an important step toward developing targeted prevention strategies for the growing population of diabetes patients at elevated risk of developing Alzheimer’s disease. We are now working to validate these findings through clinical investigations focused specifically on high-risk populations.
The preprint is available at: https://doi.org/10.1101/2025.03.14.643306
Dr. Zanos presents our latest findings at University of Athens Neuroscience Seminar Series

Dr. Zanos, the Head of the Translational Neuropharmacology Lab, had the honor of presenting his research at the University of Athens as part of their Neuroscience Seminar Series.
In his talk titled “Decoding ketamine: Neurobiological mechanisms underlying its rapid antidepressant efficacy,” Dr. Zanos shared his team’s findings on ketamine’s unique properties as an antidepressant. He explained how ketamine differs from traditional treatments by producing relief from depressive symptoms within hours rather than weeks, though its widespread clinical use remains limited by side effects.
Dr. Zanos presented evidence challenging the conventional understanding that ketamine works primarily through NMDA receptor inhibition. His research demonstrates that other NMDAR antagonists such as MK-801 do not produce the same sustained antidepressant effects, pointing to a more nuanced mechanism.
During the seminar, he highlighted the significance of the (2R,6R)-hydroxynorketamine (HNK) metabolite, which exhibits antidepressant effects without ketamine’s typical side effects. Dr. Zanos discussed the surprising inverted U-shaped dose-response relationship his lab has discovered, suggesting that some level of NMDAR activation, rather than complete inhibition, may be optimal for antidepressant efficacy.
The presentation emphasized how both ketamine and (2R,6R)-HNK require AMPA receptor activation to exert their antidepressant effects, leading to synaptic potentiation and upregulation of specific AMPA receptor subunits. Furthermore, Dr. Zanos identified the NMDAR subunit GluN2A as playing an essential role in these processes.
The University of Athens faculty and students engaged in a productive discussion following the seminar, exploring the implications of this research for developing next-generation rapid-acting antidepressants with improved efficacy and reduced side effects.
New Publication: (2R,6R)-hydroxynorketamine prevents opioid abstinence-related negative affect and stress-induced reinstatement
The Zanos lab has just published a new study in the British Journal of Pharmacology demonstrating that the biologically-active metabolite of ketamine, (2R,6R)-hydroxynorketamine (HNK) shows potential for possibly addressing multiple aspects of opioid use disorder.
In this comprehensive investigation, we established several mouse models that mimic some of the human endophenotypes of opioid dependence and withdrawal. Our findings reveal that (2R,6R)-HNK effectively prevents conditioning to sub-effective doses of morphine in stress-susceptible mice and blocks conditioned place aversion induced by precipitated withdrawal in opioid-dependent mice.
Notably, a single administration of (2R,6R)-HNK reversed anhedonia, anxiety-like behaviors, and cognitive impairment that typically emerge during protracted opioid abstinence. The compound also facilitated extinction of opioid conditioning and prevented stress-induced reinstatement of drug-seeking behaviors.
Mechanistically, we found that (2R,6R)-HNK’s therapeutic effects involve restoration of impaired cortical high-frequency EEG oscillations through a GluN2A-NMDA receptor-dependent mechanism, addressing synaptic plasticity deficits induced by chronic opioid exposure and abstinence.
This research holds particular significance as (2R,6R)-HNK is currently advancing through Phase II clinical trials. The potential to repurpose this compound for addressing stress-related aspects of opioid use disorder could accelerate the development of much-needed new treatment options in the face of the ongoing opioid crisis.
The complete study, titled “(2R,6R)-hydroxynorketamine prevents opioid abstinence-related negative affect and stress-induced reinstatement in mice,” is now available in the British Journal of Pharmacology at:
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.70018
Upcoming Seminar: “Decoding ketamine: Neurobiological mechanisms underlying its rapid antidepressant efficacy”

We are pleased to announce an upcoming seminar by Dr. Zanos, the Director of the Translational Neuropharmacology Lab at the Center for Applied Neuroscience & Department of Psychology of the University of Cyprus.
In this seminar, Dr. Zanos will discuss how unlike traditional monoamine-based antidepressants that require weeks to exert effects, ketamine alleviates depression within hours, though its clinical use is limited by side effects. While ketamine was initially thought to work primarily through NMDA receptor (NMDAR) inhibition, his research reveals a more complex mechanism. Dr. Zanos will demonstrate that NMDAR inhibition alone cannot explain ketamine’s sustained antidepressant effects, as other NMDAR antagonists like MK-801 lack similar efficacy.
Instead, the (2R,6R)-hydroxynorketamine (HNK) metabolite appears critical, exhibiting antidepressant effects without ketamine’s side effects. Paradoxically, his findings suggest an inverted U-shaped dose-response relationship where excessive NMDAR inhibition may actually impede antidepressant efficacy, while some level of NMDAR activation is necessary. The antidepressant actions of ketamine and (2R,6R)-HNK require AMPA receptor activation, leading to synaptic potentiation and upregulation of AMPA receptor subunits GluA1 and GluA2. Furthermore, NMDAR subunit GluN2A appears necessary and possibly sufficient for these effects.
This research establishes NMDAR-GluN2A activation as a common downstream effector for rapid-acting antidepressants, regardless of their initial targets, offering promising directions for developing next-generation antidepressants with improved efficacy and reduced side effects.
Learn more about our work on the lab’s Instagram page (@neuropharmlab).
Psychedelics as promising treatments for psychiatric disorders: Dr. Zanos’ article in Cyprus press

Dr. Panos Zanos, head of the Zanos Lab at the University of Cyprus, recently published an article in the Cyprus press, highlighting the therapeutic potential of psychedelics for treating psychiatric disorders. The article explores how substances like psilocybin, MDMA (ecstasy), and ketamine are emerging as innovative treatments for conditions such as depression and PTSD, particularly for patients who do not respond to conventional therapies.
Despite historical stigma and legal restrictions, modern research suggests that psychedelics may revolutionize psychiatry, offering long-lasting therapeutic effects even after a single dose. However, scientific and regulatory challenges remain, requiring rigorous clinical trials, expert supervision, and legal adaptations before these treatments can become widely available.
The article also emphasizes the need for further research in Cyprus, highlighting how funding, clinician education, and policy reforms could enable the country to participate in this rapidly growing field.
Dr. Andrea Georgiou delivers expert talk on smoking addiction biomarkers at KES College

We’re excited to announce that Dr. Andrea Georgiou, head of the Genetics Epidemiology Unit within the Zanos lab was invited to give a talk on her research on biomarkers associated with smoking addiction at KES College in Cyprus. The presentation focused on investigating biological indicators related to smoking dependence in the Cypriot population.
Dr. Georgiou brings extensive expertise to this research area, with her background in Molecular Biology and Genetics from the Democritus University of Thrace and an MSc in Advanced Biomedical Science from Imperial College London. Her current research focuses on genetic and molecular therapeutic approaches for addiction, employing advanced methodologies including Mendelian Randomization.
The seminar highlighted the ongoing work in our lab examining the biological mechanisms underlying addiction, particularly focusing on smoking behavior. This research contributes to our broader understanding of addiction mechanisms and potential therapeutic interventions.
Dr. Zanos was invited to discuss groundbreaking ketamine clinical trial on Cyprus Broadcasting Corporation
We are pleased to announce that Dr. Zanos was recently invited to appear on the Cyprus Broadcasting Corporation (RIK) to discuss our laboratory’s groundbreaking clinical trial investigating ketamine as a treatment for opioid use disorder (OUD).
This pioneering study—the first of its kind in Cyprus—is being conducted at the “GEFYRA” (BRIDGE) Substitute Substance Unit in collaboration with the State Health Services Organisation in Cyprus (OKYPY). Designed as a double-blind, placebo-controlled trial, it aims to evaluate ketamine’s potential in preventing relapse and prolonging abstinence in OUD patients, particularly those with co-occurring depression.
This research marks a significant milestone for clinical studies in Cyprus, paving the way for future innovative trials in the country. By addressing the complex challenges faced by individuals with OUD and comorbid depression—an often underdiagnosed and undertreated condition that can severely impact recovery—our trial seeks to fill a crucial gap in treatment strategies.
Through this rigorous investigation, funded by the Research and Innovation Foundation, our laboratory is advancing evidence-based treatments for substance use disorders while establishing a foundation for future clinical research initiatives in Cyprus.
We look forward to sharing updates as this important study progresses. This research exemplifies our commitment to developing novel therapeutic approaches for complex psychiatric conditions and expanding the frontiers of clinical research in Cyprus.
For more details, visit the official clinical trial registry: EU Clinical Trials Register – Ketamine for OUD Study.
New special issue led by our lab: Climate-Driven Effects on the Human Microbiome and Public Health
We’re excited to announce that our laboratory is leading a special issue in Microorganisms journal focusing on the intersection of climate change and the human microbiome. Dr. Zanos and Dr. Onisiforou are serving as Guest Editors for this initiative.
Our special issue aims to explore how environmental changes affect human-associated microbial communities and their subsequent impact on public health. We welcome submissions examining various aspects of this relationship, from direct effects on microbial ecosystems to broader public health implications.
Key research areas include:
- Climate effects on human microbiomes (gut, skin, oral, respiratory)
- Emerging infectious diseases
- Environmental factors and microbial community dynamics
- Diet-microbiome interactions in climate-affected populations
- Microbiome adaptation and therapeutic potential
The submission deadline is November 30, 2025. For questions about potential submissions or collaboration opportunities, please reach out to our team.
Contact information:
- Dr. Anna Onisiforou, Head of the Systems Bioinformatics Unit – onisiforou.anna@ucy.ac.cy
- Dr. Panos Zanos, Director of the Translational Neuropharmacology Lab – zanos.panos@ucy.ac.cy.
The Zanos Lab welcomes our new Marie Skłodowska-Curie (Onisilos co-fund) fellow, Dr. Polymnia Louka!

We are excited to welcome Dr. Polymnia Louka to the Translational Neuropharmacology Lab as an ONISILOS Marie Skłodowska-Curie Fellow. Her research focuses on the effects of ketamine’s enantiomers and active metabolites on depression-induced bone impairment, aiming to uncover novel therapeutic interventions at the intersection of neuropharmacology, bone biology, and imaging.
Dr. Louka has a diverse background in medical engineering, advanced neuroimaging, and bone biology, with expertise in bone physiology, Micro-CT imaging, and musculoskeletal research. Before joining the lab, she worked in both academia and the pharmaceutical industry, managing clinical trials and contributing to EU-funded research projects.
Welcome Dr. Louka to the Zanos lab!
Cover story feature: Zanos Lab’s groundbreaking paper on microbial influences in Alzheimer’s Disease
We are delighted to announce that our recent publication on microbial influences in Alzheimer’s disease has been selected for the cover story for Volume 13, Issue 1 (January 2025) of Microorganisms journal. This recognition by the journal highlights the significance and timeliness of our research in challenging traditional paradigms of Alzheimer’s Disease treatment.
The cover image, featuring our figure illustrating the complex interactions between microbial communities and AD pathogenesis, represents our comprehensive analysis of how various microbial factors, including the gut-brain axis, oral microbiome, brain biofilms, and viral infections, may contribute to the development and progression of Alzheimer’s disease.
This work, led by Dr. Anna Onisiforou (Head of our Systems Bioinformatics Unit in Cyprus), not only questions the limitations of the traditional amyloid cascade hypothesis but also opens new avenues for therapeutic interventions through microbiome-based approaches. The selection of our review as the cover story underscores the growing recognition of microbial factors as crucial elements in understanding and potentially treating Alzheimer’s disease.
Read the full article at: https://www.mdpi.com/2076-2607/13/1/90
Public lecture on stress and mental health coordinated by Dr. Zanos

Dr. Panos Zanos coordinated a public lecture on stress and mental health on January 23rd at the Salaminio Free/Open University of Famagusta. The event featured presentations by Dr. Maria Tsiarli, Ph.D. in Developmental Neurobiology, on “Stress and Nervous System Health,” and Professor Georgia Panayiotou, Dean of the Graduate School at the University of Cyprus, on “Psycho-emotional Life Skills and Their Role in Well-being.”
Dr. Zanos led discussions connecting neurobiological stress mechanisms with psychological impacts. The event drew significant public interest, demonstrating the importance of understanding the relationship between neurological and psychological aspects of stress in life.
Our postdoctoral fellow, Dr. Andria Michael, awarded a YUFE4Postdocs fellowship with €167,880 in funding
We are excited to announce that Dr. Andria Michael, a postdoctoral fellow in the Zanos Lab, has been awarded the highly competitive YUFE4Postdocs Fellowship, co-funded by the European Union’s Marie Skłodowska-Curie Actions (MSCA) program. This fellowship provides Dr. Michael with a 26-month research position at the University of Cyprus, offering exceptional training opportunities and a robust international research network.
Dr. Michael’s project, titled “Exploring the Role of Neurotrophic Growth Factors in Comorbid Substance Use and Mood Disorders”, aims to uncover novel mechanisms underlying protracted opioid withdrawal and its associated maladaptive behaviors, with the ultimate goal of developing more effective treatments for opioid addiction. Her interdisciplinary research combines neuropharmacology, translational neuroscience, and genetics, addressing an urgent public health challenge: the opioid epidemic.
This fellowship will also facilitate cross-institutional collaboration within the YUFE Alliance and further enhance Dr. Michael’s career prospects through its comprehensive training program.
Congratulations to Dr. Michael on this outstanding achievement!
Zanos Laboratory secures major grant funding of €600,000
We are delighted to announce that the Zanos Laboratory has secured €600,000 in research funding by the Research and Innovation Foundation, to support several innovative research programs in neuroscience and mental health. This funding will enable our laboratory to pursue multiple distinct lines of investigation that address important questions in mental health research.
In collaboration with Dr. Polymnia Georgiou, we will investigate the role of estrogen receptors in stress susceptibility to develop depression-like behaviors. Using cutting-edge in vivo CRISPR/Cas9 technology, we will examine how these receptors in specific brain circuits influence vulnerability to stress. Through advanced in vivo fiber photometry and slice electrophysiology techniques, we will further study the underlying mechanisms. Additionally, we will explore whether pharmacological activation of these receptors could reverse stress-induced behavioral changes in both male and female rodents.
In a separate research program, we will evaluate novel therapeutic approaches for addressing substance use disorders. Using intravenous drug self-administration models in rodents, we will assess the efficacy of compounds identified to have efficacy by Dr. Anna Onisiforou, the Director of our Systems Bioinformatics unit to prevent or reverse abstinence-associated negative affect and stress-induced reinstatement of drug-seeking behaviors during abstinence.
Our laboratory will also conduct research to understand the downstream molecular mechanisms of novel rapid-acting antidepressants, with a specific focus on markers of synaptic plasticity. Through electrophysiological recordings and molecular analyses, we will characterize how novel rapid-acting antidepressant compounds influence synaptic transmission and neural activity to assist with treating human depression.
These research programs will provide valuable training opportunities for researchers while generating important translational insights into mental health treatment. We look forward to advancing our understanding of these critical health challenges.
For more information about our research or opportunities to join our team, please contact the Zanos Laboratory.
New publication from the Zanos Lab: Microbial Influences in Alzheimer’s Disease
For decades, Alzheimer’s disease (AD) research has been dominated by the amyloid cascade hypothesis, which positions amyloid-beta (Aβ) accumulation as the primary driver of the disease. However, the consistent failure of Aβ-targeted therapies to demonstrate efficacy, coupled with significant safety concerns, has underscored the critical need to rethink our approach to AD treatment.
In our latest comprehensive review published in Microorganisms, led by Dr. Anna Onisiforou, we challenge this traditional perspective by exploring an emerging paradigm: the role of microbial factors in AD pathogenesis. Our analysis synthesizes mounting evidence suggesting that microbial infections may serve as crucial environmental factors in AD pathoetiology, examining the complex interactions between the gut microbiome, oral bacteria, brain biofilms, and viral infections in disease development and progression.
While a definitive causal link remains to be established, the collective evidence presents a compelling case for shifting our focus beyond the conventional amyloid hypothesis. This review not only highlights the potential mechanisms through which various microbial communities might influence AD pathogenesis but also explores promising microbiome-based therapeutic approaches that could revolutionize AD treatment strategies.
Read the full article at:
https://www.mdpi.com/2076-2607/13/1/90
2024
Dr. Zanos presents groundbreaking research on novel therapeutic approaches for opioid addiction

We are pleased to announce that Dr. Zanos recently delivered an impactful presentation on innovative approaches to treating opioid addiction. The lecture highlighted cutting-edge research developments and provided insights into the PROUD study, a pioneering clinical trial investigating ketamine’s potential in preventing opioid relapse.
During this enlightening session, Dr. Zanos shared important findings about the complex relationship between opioid addiction and affective disorders, emphasizing the critical need for more effective therapeutic interventions. Attendees learned about the limitations of current opioid substitution treatments and the promising potential of novel pharmacotherapies.
A significant portion of the presentation focused on the PROUD study – the first drug-interventional clinical trial of its kind in Cyprus. Dr. Zanos discussed how this groundbreaking research explores ketamine’s efficacy in preventing relapse among individuals with opioid use disorder, particularly during extended abstinence periods. The presentation provided valuable insights into the study’s innovative approach to identifying biomarkers that may predict relapse vulnerability and treatment efficacy.
This public engagement event reflects our ongoing commitment to advancing addiction treatment through rigorous scientific research while ensuring effective dissemination of our findings to both the scientific community and the general public.
The Translational Neuropharmacology (Zanos) Lab participates at the Center for Applied Neuroscience 2024 Conference

We are excited to share that Dr. Zanos, Director of the Translational Neuropharmacology Lab, delivered an engaging talk at the 14th Annual Scientific Conference of the Center for Applied Neuroscience (CAN), held at the University of Cyprus. His presentation, titled “From Whiskers to Wisdom: How Rodent Models can Reveal Neural Mechanisms Underlying Decision-Making and Cognitive Processes”, explored how innovative animal models advance our understanding of cognitive and decision-making processes.
The Zanos Lab was also represented by five posters, showcasing the breadth of our research:
- Dr. Anna Onisiforou presented two posters:
- “Dissecting the Contribution of Herpesviruses in the Emergence of Depressive Symptoms in Alzheimer’s Disease Through Multi-Omics Data.”
- “From Viral Infections to Alzheimer’s Disease: Unveiling the Mechanistic Links Through Systems Bioinformatics.”
- Dr. Andrea Georgiou highlighted shared genetic bases between mucosa-associated lymphoid tissue inflammation and psychiatric disorders.
- Dr. Andria Michael focused on innovative treatments to prevent negative affect and relapse during opioid abstinence.
- Dr. Eleftheria Charalambous explored the gut virome’s role in brain aging.
We extend our gratitude to the CAN organizers for creating a platform to share our work and foster collaborations within the neuroscience community.
Dr. Panos Zanos was selected as a member on the Education and Training Committee of the American College of Neuropsychopharmacology (2025–2027)

We are thrilled to announce that Dr. Zanos, the Director of the Translational Neuropharmacology Lab, has been invited to serve on the Education & Training Committee of the American College of Neuropsychopharmacology (ACNP) for the term 2025–2027. This prestigious invitation comes directly from ACNP President-Elect, Prof. William A. Carlezon, recognizing Dr. Zanos’s significant contributions to neuropharmacology and dedication to advancing the field.
The ACNP is committed to advancing scientific understanding and treatment of psychiatric and neurological disorders. Membership and participation in its committees play a crucial role in shaping the future of education and training in neuropsychopharmacology.
Dr. Zanos’s responsibilities will include:
- Mentoring early-career scientists during the ACNP Annual Meetings, offering guidance, career advice, and insights into the field.
- Reviewing travel awardee applications each year, which support early-career researchers in attending the Annual Meeting of ACNP.
- Participating in four committee meetings annually to shape educational and training initiatives in the field of Neuropsychopharmacology.
This honor reflects the Zanos Lab’s ongoing commitment to excellence in translational neuroscience and neuropharmacology research.
We look forward to seeing how Dr. Zanos’s creative ideas and expertise will shape ACNP’s educational mission and further strengthen our field.
Exciting news: Dr. Zanos establishes the Cyprus Neuroscience Society (C.N.S.)! Registration Number: ΛΕΥ|Σ|0438

Dr. Panos Zanos, the Director of the Translational Neuropharmacology Lab, proudly announces the establishment of the Cyprus Neuroscience Society (C.N.S.)—a pioneering achievement for the neuroscience-related scientific community in Cyprus. Together with 20 founding members representing various neuroscience disciplines, the founding Director of the Society, Dr. Zanos collaborates with leading neuroscientists across the country to cultivate a deeper understanding of Neuroscience and its applicationsAs the founding Director of the Society, Dr. Zanos collaborates with leading neuroscientists across the country to cultivate a deeper understanding of Neuroscience and its applications.
The CNS is a non-governmental, non-profit organization based in Nicosia, Cyprus, dedicated to advancing Neuroscience education, research, and public awareness. This Society aims to position Cyprus as a hub for neuroscience excellence, fostering both local and international collaboration.
Key Objectives of the Cyprus Neuroscience Society:
- Advancing Neuroscience Research: Supporting innovative studies that delve into the complexities of the nervous system, from molecular mechanisms to behavioral outcomes.
- Enhancing Neuroscience Education: Promoting neuroscience literacy through seminars, workshops, and reliable scientific communication tailored to students, professionals, and the general public.
- Raising Public Awareness: Engaging the community in understanding the implications of neuroscience research for health, disease, and daily life, while advocating evidence-based practices.
- Global Neuroscience Representation: Acting as a bridge between Cyprus and the international neuroscience community by participating in global conferences and initiatives.
- Empowering Neuroscientists in Cyprus: Providing resources, recognition, and networking opportunities for members to flourish in their professional efforts.
The Society also plans to organize scientific conferences/meetings, foster collaborations among neuroscientists, and publish findings that highlight the significance of Neuroscience advancements in Cyprus and beyond. Its emblem, a neuron encircled by the founding year “2024,” represents the Society’s mission to unify and elevate Neuroscience research in Cyprus.
This is an exciting milestone for Neuroscience in Cyprus, laying the foundation for collaborative efforts to unravel the mysteries of the brain and nervous system.
Stay connected for updates on our initiatives, events, and opportunities to join the Cyprus Neuroscience Society!
Dr. Zanos appointed as a member to the Cyprus National Bioethics Committee

We are thrilled to announce that Dr. Panos Zanos, the Principal Investigator of the Translational Neuropharmacology Lab, has been appointed as a member of the Cyprus National Bioethics Committee (CNBC) following a rigorous application and interview process.
The CNBC plays a pivotal role in advising and guiding ethical practices in research, healthcare, and policy-making in Cyprus. Dr. Zanos’s expertise in neuropharmacology and his commitment to advancing ethical standards in neuroscience research will contribute to the committee’s mission of promoting responsible scientific progress.
For more information about the CNBC, visit https://www.bioethics.gov.cy.
Celebrating excellence: Our Ph.D. students, Despina Melanthiou and Stylianos Valiantis awarded a training fellowship!


We are excited to share that Stylianos Valiantis, a talented Ph.D. student in the Translational Neuropharmacology Lab, has been awarded a fellowship to support their doctoral training! This highly competitive award, granted based on academic merit and research potential, highlights our Ph.D. students’ potential to advancing neuroscience research.
Their work focuses on uncovering novel mechanisms underlying the rapid efficacy of antidepressants, aiming to transform our understanding and treatment of depression. Their research holds immense promise for improving mental health care.
We congratulate both Stylianos and Despina on this well-deserved recognition and look forward to the groundbreaking discoveries they will undoubtedly achieve during his Ph.D. journey. Stay tuned for updates on their exciting work!
Grant Success: Dr. Andrea Georgiou, our Marie Curie Fellow, secures first independent research €150,000 grant

We are excited to announce that Dr. Andrea Georgiou, our esteemed Marie-Curie Fellow at the Zanos Lab, has secured her first grant as Principal Investigator! The Research and Innovation Foundation in Cyprus has awarded Dr. Georgiou a prestigious €150,000 grant to advance her innovative research program.
Dr. Georgiou’s funded project focuses on unraveling the molecular mechanisms underlying Parkinson’s disease, promising to contribute valuable insights to the field of neurodegenerative disorders. This research aligns perfectly with our lab’s mission to push the boundaries of neuroscience and develop novel therapeutic strategies.
This significant achievement not only recognizes Dr. Georgiou’s scientific excellence but also highlights the caliber of research conducted at the Zanos Lab. As she embarks on this new chapter of independent investigation, we are confident that her work will make substantial contributions to our understanding of neurodegenerative diseases.
Exciting news for the Zanos Lab: €100,000 funds awarded for laboratory equipment and consumables!
We are thrilled to announce that the Translational Neuropharmacology Lab (Zanos Lab) has been awarded €100,000 in state funds to support our cutting-edge research. These funds will be used to upgrade our laboratory equipment and ensure a steady supply of essential consumables, enabling us to advance our mission of understanding and developing novel therapies for neuropsychiatric and neurodegenerative disorders.
In addition to boosting our research capabilities, this grant will significantly enhance the educational experiences of our students. By providing access to state-of-the-art tools and resources, we aim to equip the next generation of scientists with hands-on training and valuable skills to excel in neuroscience and neuropharmacology research.
This funding marks an important step in enhancing our research capabilities, fostering innovative discoveries, and creating impactful solutions to improve brain health.
Stay tuned for updates as we continue to push the boundaries of neuroscience and neuropharmacology research!
Dr. Onisiforou from the Zanos lab presents novel findings at IDWeek 2024 in Los Angeles, USA

We are excited to announce that Dr. Onisiforou from our lab is currently participating in IDWeek 2024, taking place in Los Angeles, USA. IDWeek is a premier international conference bringing together researchers and clinicians to discuss the latest advancements in infectious diseases.
At the conference, Dr. Onisiforou is presenting her novel research on the molecular mechanisms linking infections to neuropsychiatric symptoms in Alzheimer’s disease. Her poster, titled “Dissecting the Contribution of Herpesviruses in the Emergence of Depressive Symptoms in Alzheimer’s Disease through Multi-Omics Data,” highlights groundbreaking findings on how herpesviruses may play a role in the onset of depressive symptoms in Alzheimer’s patients, utilizing cutting-edge multi-omics data analysis.
We are proud of Dr. Onisiforou’s continued contributions to this important area of research and her commitment to advancing our understanding of the complex relationship between infections and neurodegenerative diseases.
For more information on the conference, visit the IDWeek website.
Dr. Zanos and Dr. Onisiforou at the Science of Alzheimer’s & Infectious Diseases symposium in California, USA

We are pleased to share that Dr. Zanos and Dr. Onisiforou from our lab recently participated in the Science of Alzheimer’s & Infectious Diseases Symposium, hosted by the IDSA Foundation. The symposium focused on the emerging connections between infectious diseases and Alzheimer’s disease (AD), bringing together leading experts to discuss the latest scientific advancements in the field.
At the event, Dr. Onisiforou presented her novel findings on the role of infections in the development and progression of Alzheimer’s disease. Her research provides valuable insights into how infectious agents may contribute to the onset and exacerbation of AD, potentially paving the way for novel therapeutic strategies targeting infection-related pathways.
We are proud of Dr. Onisiforou’s contributions to this critical area of study and look forward to the continued impact of her work in advancing our understanding of Alzheimer’s disease.
For more information on the symposium, visit the IDSA Foundation website.
Congratulations to Dr. Polymnia Louka for securing a Marie-Curie co-fund grant to work in the Zanos lab
 We are excited to announce that Dr. Polymnia Louka, our new postdoctoral researcher, has successfully secured €150,000 in funding through the European Marie-Curie Co-Fund. The grant will support her research project titled “Identifying the Effects of Ketamine and Its Metabolites on Depression-Induced Impairment in Bone Health.”
We are excited to announce that Dr. Polymnia Louka, our new postdoctoral researcher, has successfully secured €150,000 in funding through the European Marie-Curie Co-Fund. The grant will support her research project titled “Identifying the Effects of Ketamine and Its Metabolites on Depression-Induced Impairment in Bone Health.”
Dr. Louka’s project aims to address a critical gap in understanding the connection between depression and bone health, particularly how rapid-acting antidepressants like ketamine can impact bone physiology. Her research will explore the effects of ketamine and its metabolites on bone remodeling, both in vitro and in vivo, and investigate potential synergistic benefits of combining ketamine with other agents such as sulforaphane.
This interdisciplinary study will be conducted in collaboration with the University of Cyprus and the Royal Veterinary College. Under the mentorship of Dr. Zanos and Prof. Santama, the project aims to advance our understanding of mental and physical health, potentially leading to new therapeutic approaches.
Congratulations to Dr. Louka on this remarkable achievement, and we look forward to the impactful results from this innovative research!!
New computational publication: A novel algorithm to uncover comorbid disease mechanisms
We are excited to announce the publication of our latest research in the Computational & Structural Biotechnology Journal, titled “One Path, Two Solutions: Network-Based Analysis Identifies Targetable Pathways for the Treatment of Comorbid Type II Diabetes and Neuropsychiatric Disorders.”
This study introduces a novel computational algorithm that provides unprecedented insight into the shared molecular mechanisms underlying the comorbidity of Type II Diabetes (DM2) and various Neuropsychiatric Disorders (NPDs), including Major Depression, Bipolar Disorder, Anxiety Disorder, Schizophrenia, and others.
By developing an integrated DM2 ∩ NPDs pathway-pathway network, we applied our algorithm to identify critical pathways and nodes, such as the PI3K-Akt signaling pathway, calcium signaling, MAPK, and apoptosis pathways. These insights not only reveal key targets for treatment but also pave the way for precision medicine by addressing both DM2 and NPDs simultaneously, offering potential for more effective and personalized therapeutic strategies.
The broad impact of this work lies in the versatility of the computational framework we’ve developed. Beyond DM2 and NPDs, this algorithm can be adapted to study other complex comorbid conditions, making it a powerful tool in the field of systems biology and personalized medicine.
Discover how this groundbreaking algorithm could revolutionize the approach to comorbid disease treatment:
https://www.csbj.org/article/S2001-0370(24)00331-3/fulltext
The Zanos Lab was awarded €200,000 grant to study Hydroxynorketamine’s antidepressant cellular and molecular mechanisms
We are thrilled to announce that the Zanos Lab has been awarded a grant to investigate the cellular and molecular mechanisms behind the antidepressant actions of (2R,6R)-hydroxynorketamine ((2R,6R)-HNK), in collaboration with Prof. Kobi Rosenblum. This metabolite of ketamine has shown promise in inducing rapid antidepressant effects without the adverse effects associated with ketamine itself in mice, and is now in phase II clinical trials in humans for the treatment of major depression.
The project aims to uncover the role of the eukaryotic elongation factor 2 kinase (eEF2K) pathway in mediating the sustained antidepressant actions of (2R,6R)-HNK. A multidisciplinary approach will be employed, integrating advanced techniques such as in vivo electroencephalography, ex vivo electrophysiology, immunohistochemistry, and proteome analysis.
This research has the potential to pave the way for future therapeutic developments targeting major depressive disorder, offering hope to those who have not responded to traditional treatments.
Stay tuned for more updates as this exciting project unfolds!
Dr. Zanos was invited and presented intriguing findings on ketamine’s mechanism of antidepressant action at the 11th mGlu 2024 conference in Sicily
We are excited to announce that Dr. Zanos recently presented at the prestigious mGlu 2024 meeting in Sicily, Italy. His talk, titled “Interplay between the mGluR2 signalling and ketamine’s rapid antidepressant actions: Exploring the possible role of NMDAR activation,” offered key insights into the mechanisms underlying ketamine’s antidepressant effects.
Stay tuned for more research updates from our lab. Below is a photo from the presentation!

Zanos Lab at the European Research Night 2024 in Cyprus

We are excited to share that Zanos Lab participated in this year’s European Research Night (ERN) event in Cyprus, an initiative aimed at bringing research and innovation closer to the public. During the event, we had the opportunity to engage with students and visitors, offering insights into our ongoing research projects focused on addiction.
Our team presented to students and public our two key clinical studies:
- We discussed our work on identifying biomarkers that can predict vulnerability to relapse after a period of abstinence.
- We shared our research on evaluating ketamine’s potential to prevent relapse, aiming to offer new hope in the treatment of opioid addiction.
In addition to showcasing our projects, we took the opportunity to inform the public about the significance of neuroscience research in Cyprus, emphasizing its role in advancing our understanding of brain health and addiction.
Thank you to everyone who visited our booth and showed interest in our work. We look forward to participating in future events and continuing to contribute to the scientific community.
Our postdoctoral fellow, Dr. Andria Michael, was awarded a Marie Skłodowska-Curie Young Investigator grant (€147.000) for Innovative research in the Zanos lab.

We are thrilled to announce that our postdoctoral fellow, Dr. Andria Michael, from the Zanos lab, has been awarded a prestigious Marie Skłodowska-Curie young investigator grant under the ONISILOS co-fund scheme. This grant will support Dr. Michael’s innovative research aimed at identifying the role of Vascular Endothelial Growth Factor A (VEGF-A) in the development of substance use disorders.
This cross-species project integrates cutting-edge molecular neurobiology and epigenetic methodologies, utilizing both mouse and zebrafish models to provide a comprehensive understanding of the underlying mechanisms. The study is supervised by Dr. Panos Zanos and benefits from collaborative efforts with Prof. Niovi Santama (University of Cyprus) and Prof. Debby Van Dam (University of Antwerp), bringing together expertise from multiple institutions.
By combining diverse animal models and advanced research techniques, this ambitious project aims to uncover hidden mechanisms leading to the development of substance use disorders. The findings from this study have the potential to significantly contribute to our understanding of addiction neurobiology and pave the way for next-generation, more effective treatments.
This grant not only recognizes Dr. Michael’s exceptional potential as a young investigator but also underscores the importance of innovative, multidisciplinary approaches in addressing complex neuropsychiatric conditions. We look forward to the groundbreaking insights that will emerge from this research, potentially transforming our approach to substance use disorder prevention and treatment.
New Publication: Ketamine as a Potential Treatment for Opioid Use Disorder.


The Zanos lab is excited to announce the publication of our latest research paper in the prestigious journal Biological Psychiatry. Our study explores the potential of ketamine and its metabolites as novel therapeutic strategies for treating Opioid Use Disorder (OUD).
In this comprehensive review, we examine both preclinical and clinical research on ketamine’s potential in OUD treatment. The paper discusses ketamine’s promise in managing acute withdrawal symptoms, alleviating negative affect during protracted opioid abstinence, and preventing relapse. We also delve into the molecular targets of ketamine and its metabolites, exploring their relation to OUD treatment outcomes.
Our findings suggest that ketamine and its metabolites can effectively modulate pathophysiological processes affected in OUD, offering a promising new avenue for treatment and relapse prevention. Importantly, our review indicates that ketamine and its metabolites could be tested in clinical trials for their efficacy in treating OUD. We propose that these compounds could initially serve as adjuncts to current therapies, with the potential to be developed into standalone monotherapies in the future.
For more information: https://www.biologicalpsychiatryjournal.com/article/S0006-3223(24)01591-9/fulltext
New Publication: Unveiling the Links Between Viral Infections and Alzheimer’s Disease.

The Bioinformatics Unit of the Zanos Lab is proud to announce our latest publication, “From Viral Infections to Alzheimer’s Disease: Unveiling the Mechanistic Links Through Systems Bioinformatics,” which explores the complex relationship between viral infections and Alzheimer’s disease (AD). Dr. Anna Onisiforou utilized advanced systems bioinformatics techniques to investigate how various viruses, including herpesviruses, hepatitis viruses, influenza, and SARS-CoV-2, might contribute to AD onset or progression.
Our study revealed that these viruses can impact AD-related processes through their interactions with host proteins. Herpesviruses were found to influence critical AD processes such as amyloid-β formation and neuronal death, while hepatitis viruses affected cellular homeostasis and microglia activation. Notably, we discovered that the reactivation of certain herpesviruses during SARS-CoV-2 infection could potentially enhance neurodegeneration through synergistic effects on various cellular processes.
This research highlights the intricate connections between viral infections and AD development, suggesting that different viruses may influence AD susceptibility through both shared and distinct mechanisms. Our findings open up new avenues for understanding Alzheimer’s disease and could pave the way for novel therapeutic strategies targeting viral factors that contribute to disease progression.
The full paper is available in the following link:
https://academic.oup.com/jid/article/230/Supplement_2/S128/7754707
Zanos Lab awarded €20,000 award by the A. G. Leventis Foundation for our ongoing research on rapid-acting antidepressants.
We are excited to announce that the Zanos Lab has just been awarded a grant of €20,000 by the A.G. Leventis Foundation. This funding will significantly enhance our ongoing research efforts to uncover the mechanisms of action behind rapid-acting antidepressant compounds.
Depression remains one of the most challenging mental health conditions, and the discovery of rapid-acting antidepressants has opened new avenues for treatment. Our lab is dedicated to understanding how these compounds work at a molecular level, which could pave the way for more effective therapies for those suffering from depression.
The support from the A.G. Leventis Foundation will allow us to advance our studies, contributing valuable insights to the scientific community and offering hope to millions affected by this debilitating condition.
We look forward to sharing our findings with the community as we continue our mission to make a meaningful impact in the field of mental health.
New article published in a Cypriot Newspaper by members of the Zanos Lab: Understanding “Smiling” Depression in the Era of Social Media.

Researchers from the Zanos Lab have published an article in the Greek Newspaper “Neolaia” (Youth) examining the phenomenon of “smiling” depression. This form of depression is characterized by individuals presenting a cheerful, successful image on social media while concealing their true feelings of sadness.
The article, co-authored by Dr. Anna Onisiforou and Dr. Panos Zanos, suggests that the curated, positive depictions on social media can shape perceptions and influence expectations. Algorithms that prioritize engaging content may pressure users to maintain a cheerful online persona, even when facing personal challenges.
These incidents where individuals who seemed happy on social media later took their own lives serve as a stark reminder that what we see online often doesn’t reflect the full reality of someone’s life. These tragic cases highlight the disconnect between curated online personas and the private struggles that people may be facing.
The Zanos Lab aims to raise awareness and encourage more authentic online interactions to promote individual well-being and a sense of community.
Our postdoctoral fellow, Dr. Alexis Panutsopulos was awarded a Marie Skłodowska-Curie Young Investigator Grant (€ 147.000) for Innovative Research in the Zanos Lab.

We are thrilled to announce that our postdoctoral fellow, Dr. Panutsopulos, has been awarded a Marie Skłodowska-Curie Young Investigator grant under the ONISILOS co-fund scheme. This grant will support Dr. Panoutsopoulos’ cutting-edge research aimed at investigating the role of antidepressants, such as SSRIs and ketamine, in the development of neural tube defects (NTDs) during early embryogenesis.
Dr. Panoutsopoulos’ project employs advanced methodologies using human-induced pluripotent stem cell (iPSC)-derived neural organoids to model neural tube formation and assess the effects of antidepressants on this critical developmental process. By utilizing innovative gene-editing tools such as CRISPR-Cas9, combined with molecular and proteomic analyses, the study will uncover the complex mechanisms through which antidepressants influence neural tube closure and may contribute to NTDs.
Supervised by Dr. Zanos, this interdisciplinary research is poised to provide critical insights into the safety of antidepressant use during pregnancy, particularly regarding their impact on embryonic neurodevelopment. This study not only enhances our understanding of neurodevelopmental disorders but also has the potential to influence future guidelines on the clinical use of antidepressants.
Dr. Panoutsopoulos’ award underscores his exceptional promise as a young investigator and highlights the Zanos Lab’s commitment to advancing innovative, multidisciplinary approaches to complex neurobiological challenges. We are excited about the groundbreaking contributions this research will make to the field of developmental neurobiology.
Zanos laboratory’s BioNic research featured in major Cypriot newspaper. Our research pioneers the future of smoking cessation.
 The Zanos Laboratory at the University of Cyprus has recently been featured in one of Cyprus’ leading newspapers for its groundbreaking BioNic research program. Led by Principal Investigator Dr. Panos Zanos and Marie Curie fellow Dr. Andrea N. Georgiou, this innovative study aims to revolutionize smoking cessation strategies by identifying biological markers that can predict vulnerability to relapse in individuals attempting to quit smoking.
The Zanos Laboratory at the University of Cyprus has recently been featured in one of Cyprus’ leading newspapers for its groundbreaking BioNic research program. Led by Principal Investigator Dr. Panos Zanos and Marie Curie fellow Dr. Andrea N. Georgiou, this innovative study aims to revolutionize smoking cessation strategies by identifying biological markers that can predict vulnerability to relapse in individuals attempting to quit smoking.
The BioNic program combines cutting-edge biological research with a clinically-tested avatar-based psychological intervention. By analyzing various biomarkers such as stress hormones, neurotransmitters, and epigenetic indicators associated with addiction, the research team is working towards developing more personalized and effective approaches to smoking cessation. This scientific endeavor not only promises to enhance our understanding of the biological mechanisms underlying nicotine addiction and relapse but also holds the potential to significantly improve success rates for those striving to quit smoking. The feature in the national newspaper underscores the importance and potential impact of this research on public health in Cyprus and beyond.
Groundbreaking Phase I clinical trial results: (2R,6R)-Hydroxynorketamine (HNK) shows an excellent safety profile.
We are thrilled to announce a major milestone in our research journey. The manuscript detailing the Phase I clinical trial results for (2R,6R)-Hydroxynorketamine (HNK), a metabolite of ketamine, has just been published. The study, titled “A Phase 1 Assessment of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of (2R,6R)-HNK in healthy volunteers,” demonstrates the compound’s excellent tolerability in human subjects. This publication marks a crucial moment in the trajectory of (2R,6R)-HNK, from its initial discovery from the Director of the lab, Dr. Zanos, to its current status in human clinical trials. The successful completion of this Phase I trial represents a key step in translating preclinical findings into potential therapeutic applications, which is among the important aims of our laboratory. Our collaboration with the National Institutes of Health (NIH) has been instrumental in advancing this promising compound from bench to bedside.
As this metabolite progresses to Phase II trials for both pain management and major depression treatment, we are optimistic about the potential of this compound to address significant unmet medical needs. This achievement underscores our laboratory’s commitment to innovative neuropharmacological research and its potential to improve patient outcomes.
For more details, please refer to our published manuscript: https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.3391
Stay tuned for further updates as we continue our mission to develop novel therapeutic approaches for neuropsychiatric disorders and other brain diseases.
Dr. Zanos’s Research Leads the Field in Ketamine Studies for Depression.
We are proud to announce that Dr. Zanos’s groundbreaking research on ketamine has achieved exceptional recognition within the scientific community. According to a recent publication in the European Archives of Psychiatry and Clinical Neuroscience, Dr. Zanos’s work constitutes the top three most cited papers on ketamine and its enantiomers for depression from 2000 to 2023.
- “NMDAR inhibition-independent antidepressant actions of ketamine metabolites” published in Nature (2016) has been cited more than 1500 times, highlighting its significant impact on understanding ketamine’s mechanisms beyond NMDAR inhibition.
- “Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms” published in Pharmacological Reviews (2018) with 1000 citations, offers comprehensive insights into the pharmacological profiles and therapeutic potentials of ketamine and its metabolites.
- “Mechanisms of ketamine action as an antidepressant” published in Molecular Psychiatry (2018), cited 890 times, delves into the underlying mechanisms by which ketamine exerts its rapid antidepressant effects.
These highly influential papers underscore Dr. Zanos’s leading role in advancing the field of ketamine research, providing critical insights into its therapeutic potential for treating depression and other brain diseases. His work continues to shape the scientific discourse and drive forward new avenues for clinical applications.
Dr. Zanos was invited as an expert to give an interview and answer critical question about depression and its underlying mechanisms by a groundbreaking European-funded initiative .

Dr. Zanos was invited as an expert contributor to the “TO AITION” project’s Q&A section. “TO AITION” is a groundbreaking EU-funded project investigating the complex relationship between cardiovascular disease (CVD) and depression, aiming to uncover underlying mechanisms and develop novel tools for prevention, diagnosis, and treatment. In this role, Dr. Zanos addressed critical questions about depression, leveraging his expertise in neuropharmacology. The questions covered a range of important topics, including the genetic factors in depression, the role of neurotransmitters like serotonin and dopamine, the challenges of treatment-resistant depression, and the potential of personalized medicine in improving treatment outcomes. Dr. Zanos’s selection as an expert for this initiative highlights the Zanos Lab’s significant contributions to the field and underscores their commitment to advancing understanding of depression within the context of its comorbidity with cardiovascular disease.
For more info read: https://www.to-aition.eu/qadepression
Gut-Brain connections: Our postdoctoral researcher Dr. Charalambous presents groundbreaking research on phageome and brain ageing at Grainau workshop.

Our postdoctoral researcher Eleftheria Charalambous presented our latest findings at the 12th Grainau Workshop of Genetic Epidemiology in Grainau, Germany. With her talk “The gut phageome is associated with brain ageing” she sparked insightful conversations with other researchers. Dr. Charalambous studies the link between bacteriophages in the gut microbiome and the brain. Bacteriophages are viruses that infect bacteria and are thought to be important drivers for microbiome composition and function. She particularly focuses on ageing as well as the ageing brain, investigating how these microbes could influence cognitive function over time.
Dr. Charalambous’s research indicates that the ‘gut phageome’ and the overall gut microbial ecosystem have an indispensable role in maintaining brain function and potentially manipulating age-associated neurodegenerative pathologies. The ‘gut phageome’ is defined as the totality of all bacteriophage genetic material within the gut. Dr. Charalambous’s objective is to decipher in deep detail how fluctuations in the overall phageome associate with the process of brain ageing. Based on brain ageing scores inferred from brain imaging outputs, she discovered that certain types of phages are present in healthy ageing brains, while others increase their abundance during initial stages of Alzheimer’s Diseases.
The Grainau meeting has shown us the urgency and importance of investigating microbe-host as well as gut–brain interactions, but also of developing early screening and intervention measures for age-related neurodegenerative diseases and brain health in general. This research opens up new avenues for exploring how the gut microbiome could be leveraged to promote healthy brain ageing.
Dr. Zanos discusses substance use disorders on Cyprus Broadcasting Corporation TV channel (RIK 1).
We are thrilled to share that Dr. Panos Zanos was invited to appear on RIK Sat, the satellite television channel of the Cyprus Broadcasting Corporation (CyBC), to discuss the critical issue of drug addiction. This invitation from RIK Sat, one of Cyprus’s premier broadcasting platforms, underscores the significance of Dr. Zanos’ work in the field of neurobiology and addiction, which is pivotal in a country with limited specialized research in this area.
During his appearance, Dr. Zanos provided an in-depth analysis of the neurobiological mechanisms underlying various forms of drug addiction, including opioids, stimulants, and other substances. He explained how these drugs affect the brain’s reward system, with a particular focus on dopamine—a neurotransmitter that plays a key role in reinforcing drug-taking behavior. This reinforcement is a common pathway across different substances, leading to the development of addiction.
Dr. Zanos also discussed the concept of tolerance, where repeated drug use results in the brain adapting and requiring higher doses to achieve the same effect. This cycle of increasing use and dependence often leads to severe withdrawal symptoms, which further entrench the addiction. He highlighted recent advancements in addiction research, emphasizing the importance of comprehensive treatment plans that address both the biological and psychological aspects of addiction.
The interview on RIK Sat not only shed light on the complex nature of drug addiction but also highlighted the importance of continued research and education in this field. Dr. Zanos’ insights are crucial for developing effective prevention and treatment strategies, ultimately aiming to improve outcomes for those struggling with substance use disorders.
Stay tuned to our lab’s website for more updates and insights from Dr. Zanos and his team as we continue to advance our understanding of neurobiology and addiction through cutting-edge research.
New Preprint: Network-Based Analysis Identifies Targetable Pathways in Comorbid Type II Diabetes and Neuropsychiatric Disorders.
We are pleased to announce the release of a new preprint from Dr. Anna Onisiforou and Dr. Panos Zanos, titled “Network-Based Analysis Identifies Targetable Pathways in Comorbid Type II Diabetes and Neuropsychiatric Disorders.” This preprint is available on bioRxiv and represents a significant step forward in understanding the complex interactions between Type 2 Diabetes Mellitus (DM2) and various neuropsychiatric disorders (NPDs). You can access the full preprint here.
Comorbid diseases complicate patient outcomes and escalate healthcare costs, necessitating a deeper mechanistic understanding. Neuropsychiatric disorders (NPDs) such as Neurotic Disorder, Major Depression, Bipolar Disorder, Anxiety Disorder, and Schizophrenia significantly exacerbate Type 2 Diabetes Mellitus (DM2), often leading to suboptimal treatment outcomes. The neurobiological underpinnings of this comorbidity remain poorly understood. To address this, Dr. Onisiforou and Dr. Zanos developed a novel pathway-based network computational framework that identifies critical common disease mechanisms between DM2 and the five prevalent NPDs.
Their approach involves reconstructing an integrated DM2 ∩ NPDs KEGG pathway network and applying two complementary analytical methods, including the “minimum path to comorbidity” method to identify the shortest pathways fostering comorbid development. This analysis uncovered shared pathways like the PI3K-Akt signaling pathway and highlighted key nodes such as calcium signaling, MAPK, estrogen signaling, and apoptosis pathways. The dysregulation of these pathways likely contributes to the development of DM2-NPDs comorbidity.
The findings from this study not only elucidate the intricate molecular interactions driving this comorbidity but also identify promising therapeutic targets, paving the way for innovative treatment strategies. This framework can be adapted to study other complex comorbid conditions, offering broad implications for improving patient care. The Zanos Laboratory is excited about the potential applications of this model in advancing our understanding of comorbid diseases and enhancing therapeutic approaches.
Dr. Zanos Speaks on Neurobiology of Drug Addiction at Substance Use Disorders Awareness Event in Cyprus.

We are excited to share that Dr. Zanos was invited by OFSEAK Cyprus and the Cyprus Anti-Cancer Society to deliver a keynote address on the neurobiology of drug addiction at a recent Substance Use Disorders Awareness event. This notable event took place in Cyprus and was covered extensively by several television outlets.
Dr. Zanos’s presentation discussed the neurobiological mechanisms behind drug addiction, providing critical insights into this vital area of research. The event attracted a diverse audience, including leading scientists, healthcare professionals, and policymakers. Adding to the event’s importance was the attendance of the Minister of Health of Cyprus.
New Publication: Molecular Signatures of Premature Aging in Major Depression and Substance Use Disorders.
We are excited to announce the latest publication from our talented Research Associate and Head of the Bioinformatics Unit, Dr. Anna Onisiforou, in collaboration with Dr. Polymnia Georgiou. Their groundbreaking paper, titled “Molecular signatures of premature aging in Major Depression and Substance Use Disorders,” has been published in Scientific Data (Nature Group).
Major depressive disorder (MDD) and substance-use disorders (SUDs) are known to lead to premature aging, increasing vulnerability to cognitive decline and various forms of dementia. Utilizing advanced systems bioinformatics, this study has identified specific aging “signatures” in MDD and SUDs and evaluated the potential of known lifespan-extending drugs to target and reverse these signatures. The study identified transcriptional activation of FOS gene family members as a potential target for mitigating premature aging in individuals with MDD and SUDs.
The research also highlighted that antidepressant drugs that activate the PI3K/Akt/mTOR pathway, common in rapid-acting antidepressants, may accelerate aging in MDD patients. This suggests they may not be suitable for those with aging-related conditions like dementia and Alzheimer’s disease. In contrast, several potential anti-aging interventions for MDD patients were identified, such as Deferoxamine, Resveratrol, Estradiol valerate, and natural compounds like zinc acetate, genistein, and ascorbic acid, which may be effective regardless of comorbid anxiety disorders.
These findings provide crucial insights into the premature aging effects of MDD and SUDs and offer promising treatment strategies for patients with comorbid aging-related conditions. The research underscores the importance of personalized medicine approaches in treating MDD and SUDs, particularly in patients at risk for cognitive decline and dementia. We congratulate Dr. Onisiforou and her collaborators for their significant contribution to understanding the intersection of mental health disorders and aging. This innovative work not only advances scientific knowledge but also opens new avenues for therapeutic interventions aimed at improving the quality of life for patients suffering from these complex conditions.
To read the full paper, please visit: https://www.nature.com/articles/s41597-024-03538-z.
Stay tuned for more updates and insights from the Zanos Laboratory as we continue to push the boundaries of bioinformatics and neuroscience research.
Dr. Zanos discusses the path to alcohol dependence on a TV program.
Dr. Zanos was recently invited to speak on a TV channel about the neurobiology of alcohol addiction, where he explored the complex processes that lead from casual alcohol use to full-blown dependence. Initially, alcohol consumption often presents an aversive taste; however, this negative experience is quickly overshadowed by the activation of the brain’s reward system. Central to this process is dopamine, a neurotransmitter crucial to the brain’s reward circuitry. When alcohol is consumed, dopamine levels rise, creating a sense of pleasure and reinforcing the behavior. Over time, the brain begins to associate alcohol with positive feelings, despite the initial aversion. This learned association paves the way for repeated use, as individuals seek to replicate the pleasurable effects.
As alcohol use continues, tolerance develops, necessitating the consumption of larger quantities to achieve the same euphoric effect. Dr. Zanos highlighted that this is where the dark side of alcohol addiction becomes apparent. The brain’s adaptation to alcohol results in a diminished response to its effects, driving the need for increased doses. Eventually, individuals become reliant on alcohol not for pleasure but to stave off severe withdrawal symptoms that arise when consumption ceases. These symptoms, which can include tremors, anxiety, and nausea, manifest as the brain’s dependence on alcohol to maintain normal function. Thus, what begins as spontaneous use can escalate into a cycle of dependence, where the primary motivation shifts from seeking pleasure to avoiding discomfort. Dr. Zanos’ insights underscore the importance of addressing both the neurobiological and psychological components in treatment and prevention strategies.
Our postdoctoral researcher, Dr. Eleftheria Charalambous, presented our latest findings at the 10th International Human Microbiome Consortium (IHMC) Congress 2024, held in Rome, Italy.

Her presentation, titled “The gut phageome is associated with healthy brain ageing” drew significant interest from fellow researchers. Dr. Charalambous’s work explores the connection between bacteriophages in the gut microbiome and cognitive health as we age. Her research suggests that the makeup and variety of the gut phageome may play an important role in maintaining brain function and possibly modulating age-related cognitive decline.
The positive response to this work at the IHMC Congress highlights its potential to deepen our understanding of how the gut and brain interact. It also opens up new possibilities for addressing age-related cognitive issues.
Our new chapter entitled “Neuroendocrine Regulation of Anxiety” is now out in book: Anxiety Disorders and Related Conditions.

Our latest book chapter delves into the complex interactions between the central nervous system and endocrine signals, highlighting their crucial role in shaping emotional processing and behaviors related to anxiety. While the hypothalamic-pituitary-adrenal (HPA) axis has traditionally been the focal point, this chapter expands the discussion to include various steroid and peptide hormones that are increasingly recognized for their impact on anxiety behaviors.
This comprehensive review explores recent advancements in understanding how different hormones, including those associated with brain stress systems, neuropeptides, neurosteroids, and steroid hormones, contribute to anxiety regulation. Insights from both fundamental research and clinical investigations suggest that these hormonal systems may underlie stress-induced changes in anxiety and related behaviors. By exploring these diverse mechanisms, the chapter aims to open new avenues for innovative approaches to treat stress-related mental health conditions.
The chapter identifies a range of neuroendocrine targets, offering a deeper understanding of the mechanisms underlying anxiety disorders and highlighting potential new therapeutic targets. This broadened perspective could lead to more effective strategies for mitigating anxiety and improving mental health outcomes.
For more details check: https://link.springer.com/chapter/10.1007/978-3-031-56798-8_3
Dr. Zanos was invited to present data on GluN2AR-dependent antidepressant actions of ketamine and other putative antidepressants at the CINP conference.

Dr. Zanos recently presented our latest research on the antidepressant actions of ketamine and other potential antidepressants at the European University of Cyprus. His talk focused on the role of NMDA receptor (NMDAR) activation in the rapid antidepressant effects of these compounds. Contrary to the traditional view of ketamine as an NMDAR antagonist, our findings suggest that activation of these receptors, particularly the GluN2A subunit, is crucial for their antidepressant actions.
 Our research demonstrated that ketamine and other rapid-acting antidepressants, such as (2R,6R)-hydroxynorketamine and MRK-016, depend on NMDA receptor activation to exert their effects. Behavioral pharmacology experiments, along with hippocampal protein analysis and electrophysiology studies in mice, revealed that blocking NMDA receptors inhibits these antidepressant-like behaviors. These insights highlight the potential of targeting NMDA receptor signaling to develop next-generation treatments for depression.
Our research demonstrated that ketamine and other rapid-acting antidepressants, such as (2R,6R)-hydroxynorketamine and MRK-016, depend on NMDA receptor activation to exert their effects. Behavioral pharmacology experiments, along with hippocampal protein analysis and electrophysiology studies in mice, revealed that blocking NMDA receptors inhibits these antidepressant-like behaviors. These insights highlight the potential of targeting NMDA receptor signaling to develop next-generation treatments for depression.
Dr. Zanos emphasized the broader implications of these findings for depression therapy, suggesting that enhancing NMDA receptor-dependent synaptic potentiation could be a promising strategy for new antidepressant interventions. This research opens up new avenues for developing treatments that are faster-acting and more effective for individuals with depression.
Dr. Zanos was invited to a podcast to discuss the research happening in Cyprus and its implications for human life.
Dr. Zanos recently appeared on a podcast to discuss the exciting research developments happening in Cyprus. He emphasized the importance of disseminating our findings more widely to expand research trajectories and attract more young scientists to our country. By sharing our results and fostering a collaborative scientific community, we aim to promote innovation and advance the field, ultimately benefiting both local and global communities.
Dr. Zanos presents new data on ketamine’s mechanisms of action at the European University of Cyprus.

Dr. Zanos presented our latest pre-clinical data on the antidepressant mechanisms of action of ketamine during an invited speech at the European University of Cyprus. Our research provides compelling evidence that indirect activation of NMDA receptors, rather than their inhibition, may be the key mechanism responsible for ketamine’s rapid antidepressant effects. This challenges the conventional understanding and opens new avenues for therapeutic intervention.
Utilizing a multidisciplinary approach that integrates behavioral pharmacology, advanced neuroscience techniques, and molecular genetics, our team is unraveling complex neural pathways and molecular processes involved in depression. These innovative studies are not only enhancing our understanding of how ketamine exerts its effects but are also paving the way for the development of next-generation pharmacotherapies.
Our findings have significant implications for the treatment of depression, offering hope for more effective and faster-acting therapies. By leveraging these new insights, we aim to create better therapeutic strategies that can provide relief for individuals suffering from depression, particularly those who have not responded to traditional treatments.
Our postdoctoral researcher, Dr. Georgiou, publishes important study on the genetic links between Mucosa-Associated Lymphoid Tissue Inflammation and psychiatric disorders.

Dr. Georgiou, a Marie Curie fellow at the Zanos lab, has published a novel study entitled “Investigating the shared genetic basis and causal relationships between mucosa-associated lymphoid tissue inflammation and psychiatric disorders”. The study employed a two-sample Mendelian randomization analysis using genetic data from over 342,000 FinnGen participants to investigate potential causal relationships between inflammation of the mucosa-associated lymphoid tissue, proxied by conditions like tonsillectomy, appendectomy and appendicitis, and psychiatric disorders such as major depressive disorder, schizophrenia, bipolar disorder and anxiety disorders. Surprisingly, their results suggest no direct causal link between these mucosal inflammation proxies and increased risk for psychiatric disorders, despite previous observational evidence linking the two. However, an intriguing moderate inverse genetic correlation was observed between tonsillectomy and major depression, highlighting the need for further research into mediating factors.
For more information, please visit: https://doi.org/10.3389/fpsyt.2024.1379922
 The Zanos Lab announces affiliation with the Center for Applied Neuroscience in Cyprus.
The Zanos Lab announces affiliation with the Center for Applied Neuroscience in Cyprus.
The Translational Neuropharmacology Lab (Zanos Lab) is announcing its formal affiliation with the Center for Applied Neuroscience (CAN) in Cyprus. This partnership marks an important milestone in our ongoing efforts to advance knowledge in the field of Neuroscience.
Becoming a member of CAN, a research center known for pioneering neuroscience research, recognizes the dedicated efforts and contributions of our team. This affiliation will provide valuable opportunities for collaborative research with leading neuroscientists in Cyprus and worldwide.
Our integration into CAN’s network will facilitate the exchange of ideas and foster an environment for scientific discussion and innovation. The resources and expertise available through this collaboration will enhance our research capabilities, allowing us to explore new areas and make meaningful contributions to Neuroscience.
New Study Led by our postdoctoral fellow, Dr. Onisiforou, uncovers sex-specific molecular signatures in Alzheimer’s Disease.

Our newly published study, led by Dr. Onisiforou and collaborators, unveils importantsex-specific molecular signatures within the hippocampus in Alzheimer’s disease (AD). Through meticulous analysis of postmortem samples, we have uncovered distinct gene expression patterns between male and female AD patients. Notably, our findings shed light on potential gender-specific mechanisms underlying AD susceptibility and progression. This research marks a crucial step towards understanding the nuanced interplay between sex and AD pathology, offering valuable insights for the development of tailored therapeutic strategies.
Read more at:
https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2024.1345498/full
Our postdoctoral researcher, Dr. Eleftheria Charalambous presents on her fascinating findings about viruses/bacteria and their association with Alzheimer’s Disease.

Our talented postdoctoral researcher, Dr. Eleftheria Charalambous gave a fascinating presentation to peers from the University Medicine Greifswald on her latest findings exploring potential links between viral/bacterial infections and Alzheimer’s disease. Dr. Charalambous’ research focuses on microbe-host interactions, including the gut-brain and oral-brain axes, and how they impact immune function, the hypothalamic-pituitary-adrenal axis, brain aging, and neurodegeneration. Leveraging her expertise in multi-omic data integration and high-dimensional analysis, she has uncovered intriguing associations between certain viral and bacterial pathogens and the development of Alzheimer’s pathology, suggesting viral infections may contribute to neuroinflammation that drive the neurodegenerative process. Through her innovative computational/bioinformatics approaches, Eleftheria aims to elucidate the mechanisms by which viruses potentially initiate or exacerbate Alzheimer’s disease progression.
The Zanos lab joins RePo4EU drug repurposing community
 The Zanos lab has joined the community of RePo4EU, an industry-level online platform dedicated to validated precision drug repurposing, with a worldwide impact. RePo4EU’s overarching objective is to position this platform as a central data hub, facilitating the exchange of crucial information, providing comprehensive training resources, fostering matchmaking opportunities, and nurturing collaborative initiatives to promote drug repurposing efforts. Check our partnership’s page here:
The Zanos lab has joined the community of RePo4EU, an industry-level online platform dedicated to validated precision drug repurposing, with a worldwide impact. RePo4EU’s overarching objective is to position this platform as a central data hub, facilitating the exchange of crucial information, providing comprehensive training resources, fostering matchmaking opportunities, and nurturing collaborative initiatives to promote drug repurposing efforts. Check our partnership’s page here:
https://repo4.eu/portfolio/the-translational-neuropharmacology-laboratory-university-of-cyprus/
The Zanos lab welcomes our new ONISILOS Marie Sklodowska Curie fellow, Dr. Andrea Georgiou.
Her proposed case-control study (participants that achieved abstinence for at least 6 months after smoking cessation versus participants that did not achieve abstinence) aims to identify novel genetically-supported drug targets for smoking cessation and to correlate/associate changes in those druggable protein targets with: (a) motivation to quit smoking and (b) vulnerability to relapse following abstinence.
For more information, please visit:
2023
GRANT SUCCESS: Dr. Zanos secures funding (€130.000) for critical laboratory equipment acquisition.
Dr. Zanos has recently secured funding totaling €130.000, dedicated to the acquisition of crucial laboratory equipment. This financial support represents a significant investment in advancing our research capabilities. The funds will be instrumental in acquiring cutting-edge equipment, fostering innovation, and reinforcing our commitment to conducting high-impact research. This development marks a pivotal moment in the continued growth and success of the Translational Neuropharmacology Lab research initiatives, positioning the laboratory for increased productivity and breakthrough discoveries in the field.
CONGRATULATIONS: Our team member, Dr. Mary Haddad, has secured a Marie Skłodowska Curie_CoFund (Onisilos scheme) fellowship (€147.120) with Dr. Zanos as the Supervisor.
Dr. Haddad, a postdoctoral fellow starting in July 2024 in the Zanos lab, received €147,120 in funding by the European Commission to assess the Role of Cytochrome P450 Lipid Mediators in Depression and Neurodegeneration. Immunological factors, proinflammatory mediators, oxidative stress markers, and endocrine dysregulation are implicated in depression pathology. Existing therapies face challenges, urging the need for more effective and safe alternatives. This study will explore the potential of Cytochromes P450 (CYP) enzymes, specifically focusing on their role in oxidative stress and arachidonic acid metabolism. CYP-induced oxidative stress is linked to various pathologies, with limited insight into its role in depression. The study aims to investigate the neuroprotective and antidepressant potential of CYP regulation both in vitro and in vivo, with a focus on depression-relevant pathways, contributing to a better understanding of pathological nerve dysfunction, cognitive impairment, and neuronal injury in depression.
NEW BIOINFORMATICS MANUSCRIPT: From Viral Infections to Alzheimer’s Disease: Unveiling the Mechanistic Links Through Systems Bioinformatics.

Viruses Might Influence the Development of Alzheimer’s Disease!!
Scientists have long been curious about the connection between viral infections and the onset of Alzheimer’s Disease (AD), a debilitating condition characterized by memory loss and cognitive decline. Here, we used a systems bioinformatics approach to explore the potential roles of various viruses, including Herpes Simplex Virus 1 (HSV-1), Human Cytomegalovirus (HCMV), and others, in the development of AD through the interactions between virus and host proteins. We concluded that viral infections can lead to increased susceptibility for AD development. Our findings could pave the way for the identification of novel strategies to understand and intervene in the progression of Alzheimer’s Disease.
Read more at: https://www.biorxiv.org/content/10.1101/2023.12.05.570187v1
Dr. Zanos presents our latest findings on novel mechanisms underlying ketamine’s antidepressant action.

The Director of the Translational Neuropharmacology Lab, Dr. Zanos, presented our latest data concerning the potential role of NMDA receptor activation underlying ketamine’s antidepressant mechanism of action. This invited talk was a part of the Hellenic Society for Neuroscience (HSfN) conference. Dr. Zanos’s talk centered on revealing the crucial role of NMDA receptor activation in facilitating ketamine’s rapid antidepressant effects. This research illuminates potential pathways for the development of safer and more effective antidepressant interventions.
If you want to learn more about these advancements, read our new manuscript published in the Journal of Neuroscience recently: https://www.jneurosci.org/content/43/6/1038.abstract.
Stay tuned for future advancements in this exciting field.
NEW PUBLICATION: Unraveling the transcriptomic signatures of Parkinson’s Disease and Major Depression using single-cell and bulk data.

Curious to know what Parkinson’s disease and Major depression have in common? So are we! Explore our latest publication, where we analyze single-cell and bulk data to unravel their transcriptomic signatures.
https://www.frontiersin.org/articles/10.3389/fnagi.2023.1273855/full
Dr. Zanos Delivers Invited Lecture to the General Public: “Understanding the Effects of Drugs of Abuse on the Brain and Discovering Innovative Pharmacotherapies”.

We are excited to announce that Dr. Zanos was invited to deliver a special lecture to the general public, shedding light on the profound impact of drugs of abuse on the brain and presenting innovative pharmacotherapies.
In this engaging and informative presentation, Dr. Zanos shared his expertise on the subject, providing valuable insights into the neurological effects of substance abuse and the cutting-edge pharmacological approaches that may hold the key to addressing these challenges.
Our commitment to public education and raising awareness about critical issues at the intersection of neuroscience and pharmacology is at the core of our mission.
Dr. Zanos Presents Groundbreaking Research at the Mediterranean Neuroscience Society Conference.
Dr. Zanos recently presented our laboratory’s groundbreaking findings at the Mediterranean Neuroscience Society conference held in Tunis (https://www.medneuroscisociety.org/mns2023_info). His presentation focused on the novel discoveries surrounding the paradigm-shifting mechanisms responsible for the rapid-antidepressant efficacy of ketamine and other potential compounds currently in clinical phase development.
This presentation highlights the significant contributions of our laboratory to the field of neuroscience and reinforces our commitment to advancing the understanding of rapid-acting antidepressant treatments. We are proud of Dr. Zanos’ dedication and the team’s exceptional work in this important area of research.
Stay tuned for more updates on our latest research and achievements.
Dr. Andrea Georgiou receives €147,120 by the Marie Skłodowska Curie_CoFund (Onisilos scheme).
We are proud of our lab member, Dr. Andrea Georgiou, who has been awarded a competitive Marie Skłodowska Curie_CoFund fellowship, with Dr. Zanos as the Supervisor. She will be identifying genetically-supported drug targets for smoking cessation by assessing participants who have successfully maintained abstinence for at least six months. This study will also explore the correlation between these drug targets and motivation to quit smoking, nicotine dependence, and vulnerability to relapse. Potential targets will be identified using drug repurposing Mendelian randomization and confirmed by assessing gene methylation levels.
For more information, please visit: https://www.ucy.ac.cy/onisilos/wp-content/uploads/sites/169/2023/12/A.G-ONISILOS-MSCA-COFUND-FELLOW.pdf
2022
EXCITING NEWS: Drs. Zanos and Onisiforou secure $100,000 in funding from the Infectious Diseases Society of America.
In our research initiative, we will explore viral-mediated pathogenic mechanisms to understand their potential involvement in the development of comorbid Alzheimer’s Disease and Major Depression. Employing Systems Bioinformatics, our investigation will involve a thorough analysis of the complex interactions between viral agents and the molecular pathways associated with both conditions. By integrating diverse biological datasets and utilizing advanced computational techniques, we aim to unravel the complex molecular processes that may contribute to the co-occurrence of these conditions. This multidisciplinary approach, grounded in Systems Bioinformatics, holds the promise of revealing novel insights into the interplay between viral infections and the pathogenesis of comorbid Alzheimer’s Disease and Major Depression. Ultimately, our research seeks to contribute to the development of innovative therapeutic strategies and preventive interventions.
NEW GRANT: Dr. Zanos has successfully secured €199,920 in funding from the Cyprus Research and Innovation Foundation for a new project.
Exciting developments are underway in our new research initiative, where we are delving into the promising realm of drug repurposing. Specifically, our project focuses on the repurposing of ketamine to assess its efficacy in preventing relapse among patients battling with comorbid mood and opioid-use disorders. This innovative approach aims to leverage the therapeutic potential of ketamine in a novel context.
Beyond evaluating the effectiveness of ketamine in averting relapse, our study also aims to unravel the neurobiophysiological mechanisms underlying through which ketamine exerts its effects, aiming to comprehend how it prolongs periods of abstinence and acts as a formidable barrier against the resurgence of opioid dependence.
What makes our study particularly groundbreaking is that it signifies a pioneering milestone for clinical research in Cyprus. This project is the first-ever interventional clinical trial with drug administration of its kind in the country, highlighting our commitment to advancing scientific knowledge and therapeutic possibilities in the field of substance use disorders.
Dr. Zanos receives start-up funding from the University of Cyprus to enhance his research activities.
We are delighted to announce that our lab has secured a start-up funding of €50,000 from the University of Cyprus. This funding will be utilized to acquire a 128-channel EEG for the pursuit of our EU-funded clinical study, which aims to identify biomarkers of nicotine addiction.
OUR NEW PRE-PRINT: (2R,6R)-hydroxynorketamine facilitates extinction and prevents emotional impairment and stress-induced reinstatement in morphine abstinent mice.

In the realm of the opioid addiction crisis faced by many parts of the world, a significant challenge is the high relapse rates during periods of abstinence, often triggered by stress and negative emotional states. Our findings advance our understanding of opioid addiction neurobiology and treatment options by exploring the therapeutic potential of drug repurposing with (2R,6R)-hydroxynorketamine (HNK) in opioid addiction, using mice. We first established novel mouse models for examining stress susceptibility and opioid addiction interplay, uniquely enabling us to investigate HNK’s effects across various addiction-related behaviors. Our study indicates that (2R,6R)-HNK has the potential to evolve into a next-generation pharmacotherapy for opioid use disorders, which could revolutionize treatment strategies, particularly by targeting the emotional disturbances that persist through protracted abstinence.
Read more at: https://www.biorxiv.org/content/10.1101/2023.12.07.570550v1NEW PUBLICATION: NMDA Receptor Activation-Dependent Antidepressant-Relevant Behavioral and Synaptic Actions of Ketamine.

Check out our new publication in the Journal of Neuroscience: https://www.jneurosci.org/content/43/6/1038
The anesthetic and antidepressant drug ketamine is well-characterized as an NMDA receptor (NMDAR) antagonist; though, the relevance and full impact of this pharmacology to its antidepressant actions is unclear. We found that NMDAR activation, which occurs downstream of their initial actions, is necessary for the beneficial effects of ketamine and several other putative antidepressant compounds. As such, promoting NMDAR signaling, or other approaches that enhance NMDAR-dependent long-term potentiation (LTP)-like synaptic potentiation in vivo may be an effective antidepressant strategy directly, or acting synergistically with other drug or interventional treatments.
2021
GREAT NEWS: Dr. Zanos receives a prestigious Marie Skłodowska Curie Individual Fellowship (€145,941) to identify novel biomarkers predicting nicotine relapse.
Our project aims to provide insights into nicotine addiction and improve treatment outcomes. By analyzing heart rate variability (as a proxy of emotion regulation ability) in response to psychosocial stress, our team aims to investigate whether emotion regulation ability prior to nicotine abstinence can have predictive implications for maintaining abstinence. Additionally, the study will assess changes in neural activity using EEG spectral analysis and coherence between brain regions to identify potential indicators of relapse vulnerability in chronic smokers undergoing psychosocial stress before quitting. The investigation also includes an examination of EEG oscillations during a 24-hour abstinence from smoking to explore their potential role in predicting treatment outcomes. Lastly, the project will evaluate the predictive value of reciprocal interactions between stress reactivity, emotion regulation ability, and synchronized neural activity prior to smoking cessation in determining nicotine relapse vulnerability. This research approach is expected to greatly contribute to our understanding of addiction mechanisms and enhance the development of more effective smoking cessation strategies.
2018
BREAKING NEWS: Dr. Zanos awarded $70,000 Brain and Behavior Research Foundation Young Investigator Grant.
We are thrilled to announce that Dr. Zanos has been awarded a prestigious $70,000 Brain and Behavior Foundation Young Investigator Grant. The grant will support his groundbreaking research project titled, “Effects of Ketamine’s Metabolite (2R,6R)-HNK on Opioid Withdrawal-Induced Emotional Impairment and Drug-Seeking Reinstatement” (NARSAD Young Investigator Grant). This initiative holds promise for unraveling critical insights into the complex interplay between ketamine’s metabolite and emotional impairment during opioid withdrawal, paving the way for innovative strategies in addressing drug-seeking reinstatement. Dr. Zanos’ dedication to advancing our understanding of neuropsychiatric disorders is further underscored by this significant recognition from the Brain and Behavior Research Foundation.





















